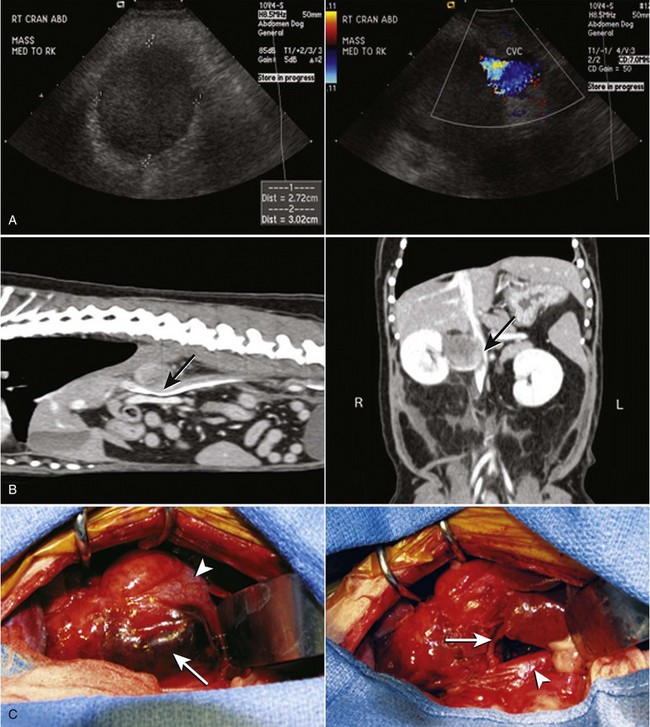
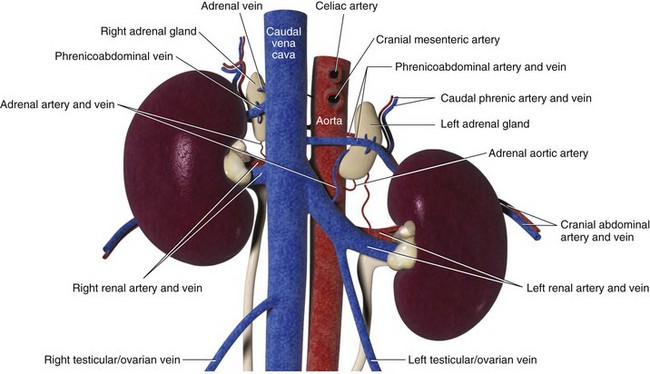
Chapter 120 The widespread availability of abdominal ultrasonography has led to increased detection of adrenal masses in human and veterinary medicine. In fact, frequent diagnosis of adrenal masses unrelated to the primary complaint has resulted in a new term in both disciplines—the adrenal incidentaloma.27 Unfortunately, the complex anatomy and physiology of the adrenal gland present a variety of challenges at every level, from diagnosis of disease conditions to decision making, perioperative care, and surgery itself. Thus, modern veterinary surgeons are often faced with the difficult task of weighing the risks and benefits of elective adrenalectomy, a procedure with significant perioperative mortality. Despite challenges associated with surgery, it is generally agreed that adrenalectomy is indicated in animals with functional tumors and those with characteristics of malignancy.27 In these instances, proper knowledge of adrenal physiology and perioperative care can improve the chances of a successful patient outcome.19 The paired adrenal glands are located in the retroperitoneal space, closely associated with the aorta and vena cava in the cranial abdomen (Figure 120-1).20 The left adrenal gland is located medial to the cranial pole of the left kidney and is loosely adhered to fascia of the psoas minor muscle and transverse process of the second lumbar vertebra. The left adrenal gland is adjacent to the left side of the abdominal aorta medially, and its caudal aspect borders the left renal artery. The right adrenal, which is further cranial than the left, is located ventral to the thirteenth thoracic vertebra and is adhered to the right side of the vena cava. In many instances, the adrenal capsule is actually contiguous with vascular adventitia.20 The right adrenal gland is covered by the caudal extension of the right lateral liver lobe; access to the region can be further complicated by hepatomegaly that accompanies hyperadrenocorticism. Both adrenal glands are also obscured by adipose tissue that accumulates in this region of the retroperitoneal space; however, they are easily identified by the beige appearance of the adrenal cortical tissue and by the phrenicoabdominal vein that crosses the ventral surface of each gland. Figure 120-1 Regional and vascular anatomy of the paired adrenal glands in a dog. (Illustration by Tim Vojt.) The microscopic anatomy of the adrenal glands reflects developmental origin and the physiologic function of this endocrine organ. The adrenal cortex is derived from a mass of mesodermal cells that arise near the genital ridges during embryonic development.20 These cells differentiate into polygonal to columnar shapes, with varying lipid content.20 The cortex takes on a laminar architecture adapted to serve specific endocrine functions, including regulation of renal fluid and electrolyte balance (aldosterone synthesis) and chronic stress adaptation and carbohydrate metabolism (steroid hormone synthesis). This mesodermal mass is later invaded by neural crest ectoderm, which migrates to the center of the gland and forms the adrenal medulla.20 As indicated by its developmental origin, the adrenal medulla is essentially a sympathetic ganglion, consisting of postsynaptic neurons that are modified to release their neurotransmitters (epinephrine and norepinephrine) into the systemic circulation through the adrenal gland’s rich vasculature. The arterial supply to the adrenal glands consists of 20 to 30 small branches arising from the phrenicoabdominal, renal, and cranial abdominal arteries and directly from the adjacent aorta.20 These arteries form a plexus, which is visible through the thick adrenal capsule, and send penetrating branches into the cortex and medulla. Venous blood is collected in sinusoids and drains into a single adrenal vein. The right adrenal vein empties directly into the vena cava, and the left adrenal vein empties into the left renal vein. Adrenal masses are most commonly identified during ultrasound examination of the abdomen and less commonly with computed tomography (CT) or magnetic resonance imaging (MRI) (Figure 120-2). Clinical signs, findings on physical examination, results of routine blood and urine tests, or a combination of these may suggest adrenal disease (e.g., hyperadrenocorticism) and the need for diagnostic imaging. Alternatively, the adrenal mass may be an unexpected finding during abdominal ultrasonography that is performed for another reason (e.g., persistent vomiting). Regardless of how an adrenal mass is discovered, determining the functional status of the mass is critical to ensure appropriate perioperative management of the case and to improve the likelihood of a successful outcome after adrenalectomy. Before proceeding with therapeutics, one of the first considerations is to confirm that an adrenal mass exists. Because of the subtlety of findings in ultrasound examination of the adrenal glands, the authors typically recommend repeating abdominal imaging to confirm that the mass is a repeatable finding and that therapy is warranted. Bulbous enlargement of the cranial or caudal pole of the adrenal gland is common in dogs with normal adrenal glands and can often be misinterpreted as an adrenal mass. The diagnosis of an adrenal mass is made when the maximum width of the adrenal gland exceeds 1.5 cm, the gland loses its typical “kidney-bean” shape, and the gland is asymmetric in shape and size compared with the contralateral adrenal gland.5,17 Adrenal tumors may secrete a hormone or may be nonfunctional. Excess secretion of cortisol, catecholamines, aldosterone, progesterone, and steroid hormone precursors have been documented in dogs and cats (Table 120-1). Clinical presentation and results of routine blood and urine tests often provide clues to the functional status of the adrenal tumor. The most common functional adrenal tumors in dogs secrete cortisol or catecholamines. Dogs with a cortisol-secreting adrenal tumor have clinical signs of hyperadrenocorticism, including polyuria, polydipsia, polyphagia, panting, abdominal enlargement, endocrine alopecia, mild muscle weakness, and lethargy.12 Findings on routine blood and urine tests include the presence of a stress leukogram, increased serum alkaline phosphatase activity, hypercholesterolemia, isosthenuria or hyposthenuria, and proteinuria.12 Abdominal ultrasonography will reveal a variably sized adrenal mass, typically ranging from 1.5 to greater than 8 cm in maximum width. The mass may compress or invade adjacent blood vessels and organs; these findings are suggestive of carcinoma (see Figure 120-2).23 Asymmetry in the size of the adrenal glands is typical. Ideally, the contralateral unaffected adrenal gland is small or undetectable (maximum width, typically <0.3 cm) as a result of adrenocortical atrophy induced by hypercortisolism. However, a normal-size contralateral adrenal gland does not rule out hyperadrenocorticism caused by an adrenal tumor. Rarely, bilateral cortisol-secreting adrenal tumors are present. The low-dose dexamethasone suppression test is used to establish the diagnosis of hyperadrenocorticism. Suppression is defined as a 4-hour postdexamethasone serum cortisol concentration below 1.5 µg/dL, a 4-hour postdexamethasone serum cortisol concentration less than 50% of the baseline concentration, or an 8-hour postdexamethasone serum cortisol concentration less than 50% of the baseline concentration.13 Serum cortisol concentrations do not suppress during the low-dose dexamethasone suppression test in dogs with adrenal-dependent hyperadrenocorticism. Failure of serum cortisol concentrations to suppress does not by itself confirm adrenal-dependent hyperadrenocorticism, however; approximately 40% of dogs with pituitary-dependent hyperadrenocorticism also fail to suppress during the low-dose dexamethasone suppression test.13 In a dog with an adrenal mass and clinical signs of hyperadrenocorticism, suppression of serum cortisol during the low-dose dexamethasone suppression test strongly supports the presence of a non–cortisol-secreting adrenal tumor (e.g., pheochromocytoma) in a dog with pituitary-dependent hyperadrenocorticism, especially if the contralateral adrenal gland is normal in size and appearance. Determination of a single baseline plasma ACTH concentration may aid in distinguishing dogs with adrenal-dependent hyperadrenocorticism from those with pituitary-dependent hyperadrenocorticism after the diagnosis of hyperadrenocorticism has been established with the low-dose dexamethasone suppression test.17,32 Dogs with a functional adrenal tumor are likely to have low (e.g., <10 pg/mL) or undetectable concentrations of endogenous ACTH. Dogs with iatrogenic hyperadrenocorticism also have low endogenous ACTH; however, these patients have subnormal baseline cortisol concentrations.
Adrenal Glands
Anatomy
Identification of An Adrenal Mass
Diagnosis of Functional Adrenal Tumors
Cortisol-Secreting Adrenal Tumors
Low-Dose Dexamethasone Suppression Test
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Adrenal Glands
Only gold members can continue reading. Log In or Register to continue