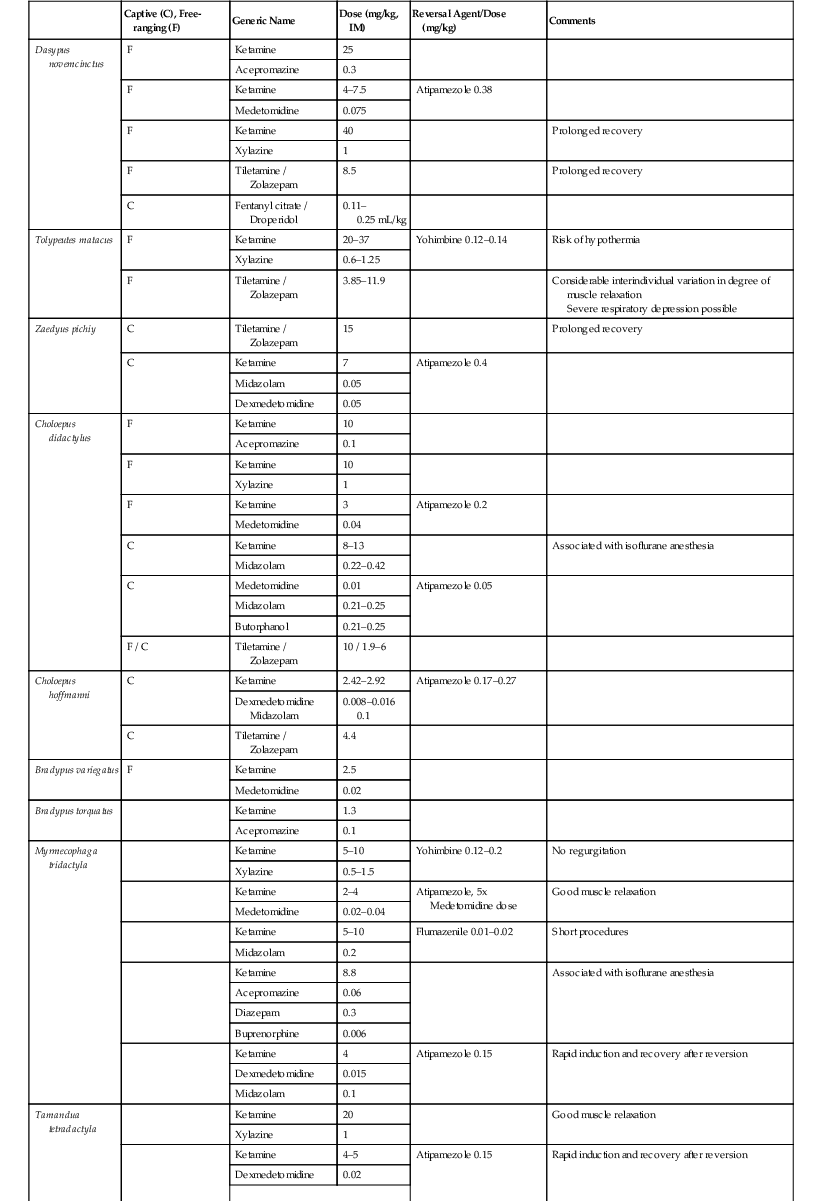
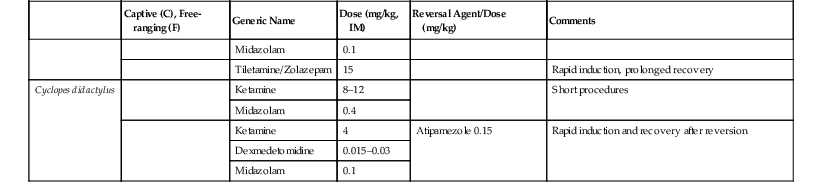
Roberto F. Aguilar, Mariella Superina The superorder Xenarthra consists of 31 extant species of armadillos (Cingulata—Dasypodidae), sloths (Pilosa—Folivora: Bradypodidae and Megalonychidae), and anteaters (Pilosa—Vermilingua: Myrmecophagidae and Cyclopedidae), which are restricted to the New World. This monophyletic group has a fossil history of at least 65 million years and is considered one of the basal clades of placental mammals.14 Most xenarthrans remain inadequately studied, and taxonomic uncertainties still exist. The 21 living armadillo species show different degrees of fossoriality and considerable differences in habitat preferences, diet, and behavior. Table 39-1 provides information on selected armadillo species. Some species such as Dasypus novemcinctus, Chaetophractus villosus, and Tolypeutes matacus are commonly kept in zoologic institutions.53 TABLE 39-1 Biologic Information on Selected Species of Armadillos a DD, Data Deficient; LC, Least Concern; NT, Near Threatened; VU, Vulnerable. Main threats: hunting, habitat fragmentation, and degradation. b Polyembryony. The two genera and six species of arboreal sloths are restricted to New World tropical rainforests (Table 39-2). The two species of two-toed sloths (Choloepus), which have predominantly nocturnal habits, are relatively common in zoologic collections. The four predominantly diurnal species of three-toed sloths (Bradypus) are only rarely kept.19,33,53 TABLE 39-2 Biologic Information on Sloths and Anteaters a CR, Critically Endangered; DD, Data Deficient; LC, Least Concern; VU, Vulnerable. Main threats: Hunting for food or pet trade, habitat degradation and fragmentation (sloths and anteaters); wildfires, persecution as pest species, road traffic (anteaters). b LC, Main population; DD, Northeastern Brazil subpopulation. All four anteater species are myrmecophagous but differ in their habits. Giant anteaters (Myrmecophaga tridactyla) are ground dwellers and have a limited ability to climb. Lesser anteaters (Tamandua spp.) are predominantly arboreal but also move, feed, and rest on the ground; and silky anteaters (Cyclopes didactylus) are strictly arboreal and nocturnal (see Table 39-2).36 The Xenarthra bear several unique anatomic traits such as additional (xenarthrous) joints of lumbar vertebrae; fusion of the ischium to the anterior caudal vertebrae; a secondary scapular spine; extensive retia mirabile in the limbs; paired postrenal venae cavae; and ossified sternal ribs. Their well-developed claws are used for digging, clinging to tree branches, and defense. Except for the toothless Vermilingua, xenarthrans have significantly reduced, homodont, continuously growing teeth that lack enamel. Caniniform teeth are present in sloths. The most conspicuous morphologic feature of armadillos is the presence of a carapace consisting of ossified dermal tissue covered by epidermal scales.22 Sloths have specialized, large stomachs consisting of several chambers, in which bacterial flora breaks down and ferments rough plant material. They descend to the ground about once a week to defecate. All Xenarthra have low basal rates of metabolism that are around 40% to 60% of those expected for their body mass.31 This should be taken into account when medicating them, as drug metabolism may be reduced. Body temperatures of Xenarthra are low and variable. In some cases, they vary according to ambient temperature.31 At least one species (Zaedyus pichiy) may enter torpor and hibernation.50 Because of their particular susceptibility to low ambient temperatures, xenarthrans should have access to a heated shelter if ambient temperature drops below 15° C. Access to both sunlight and shaded places will help them thermoregulate. Two-toed sloths tolerate temperatures from approximately 17° C to 35° C and require high humidity levels (60%–80%). Armadillos should be kept on a soft substrate such as soil, stringbark mulch, or wood shavings, which allow them to dig. Hard flooring such as concrete bears a higher risk of injury because of the armadillos’ constant digging attempts. Outdoor facilities require wall footers extending underground for at least 1 meter (m) to keep them from escaping. If kept in pairs or groups, sufficient hiding places must be provided to prevent aggression between conspecifics. Injured armadillos should be separated to prevent cannibalism. Captive sloths require large spaces with multiple areas to climb, platforms for feeding or resting, and hides. The use of ropes should be avoided as the claws may become entangled. Floors should consist of soil and be devoid of hard or sharp objects to prevent injuries in case of accidental falls.33,53 Bradypus sloths are extremely difficult to maintain in captivity because of their specialized diet, as well as their susceptibility to infectious diseases and management-related diseases. Male sloths may show aggressive behavior when defending territory.33,53 Anteaters should be kept on natural flooring. Tamandua and Cyclopes require numerous branches to climb. Myrmecophaga like to bathe and defecate in water and should thus have access to a pool. Rotting logs should be offered to allow anteaters to forage and wear down their claws. Armadillo species may be classified as carnivores–omnivores, generalist fossorial insectivores, generalist terrestrial insectivores, or specialist insectivores (anteaters and termite eaters) according to their trophic specialization.43 Captive diets consist of a mixture of beef or dog food, fruit, vegetables, eggs, and vitamin and mineral supplements. Interspecific and seasonal differences are usually not considered in captive diet composition. Pathologies related to inadequate diets are common and include obesity, renal failure, digestive imbalances, hypovitaminoses, and dental disease.53,54 The diet of wild sloths is rich in fiber and contains low levels of energy and soluble carbohydrates. Three-toed sloths (Bradypus) have a highly selective diet composed of 99% leaves, mainly of Cecropia. Individuals of this genus seem to become strongly attached to a specific tree species, and large individual differences seem to exist in the digestive efficiency. Transferring them to a captive diet is difficult.6,53 The natural diet of two-toed sloths (Choloepus) is more varied. In addition to plants, they sometimes eat insects, birds, and small reptiles.6,53 Captive individuals may eat a wide variety of food items, including fruit and vegetables, commercial small animal preparations, boiled eggs, and cheese. Leaves, flowers, and wild fruit must be offered, and the food must be placed on elevated platforms. Food items should be cut lengthwise to facilitate handling. Sources of animal protein or commercial diets for dogs or primates should not exceed 10% of the diet. Some authors believe that sloths do not drink water but, rather, obtain water from the breakdown of fresh leaves.30 Clinically, inadequate or imbalanced diets have been implicated in digestive stasis, rectal prolapse, severe diarrheas, dysbiosis, stunted growth, and urolithiasis. Anecdotal reports suggest that some sloths respond well to transfaunation during episodes of suspected dysbiosis. Anteaters forage almost exclusively on ants and termites, but the proportion of these insects varies among species, regionally, individually, and seasonally.44 Giant anteaters (M. tridactyla) may also ingest seeds, beetle larvae, and bees, and lesser anteaters (T. tetradactyla) consume beetles and seeds. Tamandua mexicana has been observed foraging on fruit of the palm Attalea butyracea.3,8,44 Several diets have been formulated for feeding Myrmecophaga and Tamandua in captivity. Diets are usually based on a semi-liquid mix that contains dog or cat food, lactose-free milk, egg, red meat, and fruit. Cyclopes didactylus only ingests arboreal ants, making the formulation of an artificial diet extremely difficult.2 All anteater species’ diets should be supplemented with vitamin K (Myrmecophaga: 5 milligrams per day [mg/day]; Tamandua: 3 mg/day) and taurine (2.2 grams per day [g/day] for Myrmecophaga and Tamandua) (Miranda, personal communication). Vitamin K deficiency may lead to hematuria and prolonged bleeding. Taurine deficiency has been associated with dilatory cardiomyopathy. Giant anteaters will develop gastrointestinal (GI) impaction and constipation or chronic soft stool and diarrhea if fiber and chitin levels in the diet are low. The diet of Cyclopes should be supplemented with selenium (0.2 g), taurine (0.18 mg), probiotics, vitamins, and minerals (Rojas Moreno, personal communication). Armadillos may defend themselves with their limbs and sharp claws when being caught. Heavy gloves should be used while holding them to avoid scratches. Euphractus sexcinctus is the only species that may try to bite. Armadillos are agile and will try to wriggle out of the handler’s grip. Applying light lateral pressure on the carapace often calms them down. Tolypeutes species roll up into a ball when feeling threatened. They will unroll themselves once they are placed on a hard surface. Large armadillos may be picked up by the lateral edges of the carapace and smaller species grasped by the carapace with one hand. They should only be held by the base of the tail, and their carapace supported with the other hand for short periods. Two-toed sloths (Choloepus) are more aggressive than three-toed sloths (Bradypus) and need to be handled more carefully. Adult two-toed sloths may grip the arms or body of the restrainer and inflict severe injuries with their sharp claws or caniniform premolar teeth. Physical restraint must be gentle but firm and performed by at least two persons using leather gloves to hold the feet and the head of the animal. Attempts to force physical restraint on a sloth are likely to be unsuccessful or even dangerous. Infants may feel more comfortable if allowed to grip the arm of a restrainer. Tamandua and Cyclopes may be held using leather gloves by grasping the forearms and head or simply placing them in a cloth bag.58 In the case of Cyclopes, using a cloth bag, to which they may cling, may facilitate manipulation and weighing. Myrmecophaga may be restrained using forked wooden sticks or nets, making sure the mouth or lips are not harmed during the capture.34,58 Special care must be taken to avoid their claws, and gauze and elastic bandages may be placed over them during procedures to avoid potential injury. Xenarthra have low metabolic rates and low and varying body temperatures. Their chemical restraint must therefore be performed at a stable room temperature. In cool environments, a heat source should be used to avoid significant drops in body temperature. Vital signs should be monitored during the entire procedure. Intubation of anteaters and armadillos may be challenging to impossible, given their peculiar anatomy and prolonged diastemas in a very narrow mouth. Intubation of Dasypus novemcinctus may be possible with a polyethylene tube of 0.6 to 1.3 cm diameter and a flexible endoscope.18 Anesthesia may be maintained with tight-fitting masks, provided secretions are not overabundant. Sloths may usually be intubated by using conventional small animal techniques and materials. Most inhalation and injectable anesthetics may be used for armadillos (Table 39-3).12,21,37,54 Considerable interspecific and individual differences have been observed in the duration and depth of anesthesia, and doses may need to be adjusted accordingly.54 Animals of the genus Dasypus seem to be less sensitive to ketamine compared with other Xenarthra and require a higher dose.21 Premedication with 0.02 to 0.04 mg/kg atropine prevents intensified salivation during anesthesia with ketamine. Inhalation anesthesia with isoflurane is recommended for longer surgical interventions. Induction with masks or in chambers may be problematic because of the armadillos’ ability to hold their breath for several minutes.54 Chemical restraint of sloths may be risky and even life threatening for the patient. Several authors recommend the use of injectable chemical restraint agents (see Table 39-3).12,24,27,45,56 Isoflurane anesthesia by means of a facemask, followed by intubation, is recommended rather than fixed anesthesia protocols. A variety of fixed anesthesia protocols has proven effective in anteaters, both in field and in captive conditions (see Table 39-3).20,34,46,58 The anesthetic may be injected by hand after physical restraint or darted into the forelimb muscles. In armadillos, the presence of the carapace precludes easy palpation or auscultation. Rectal temperature is difficult to interpret because of the highly variable body temperature of armadillos, which may range from 32° C to 38° C in healthy individuals. Blood is best collected from the ventral tail vein (Figure 39-1). The medial saphenous vein may be used as an alternative, especially for larger volumes or for catheterization.54 Normal heart rate is around 116 per minute in Chaetophractus villosus and 126 per minute in male Dasypus novemcinctus or 84 per minute in female D. novemcinctus. The respiratory rate in relaxed C. villosus and D. novemcinctus is 30 to 40 per minute; in the former species, it may rise up to 160 per minute in stress conditions, whereas rates of up to 180 per minute have been measured in the latter at high ambient temperatures.4,54
Xenarthra
Biology
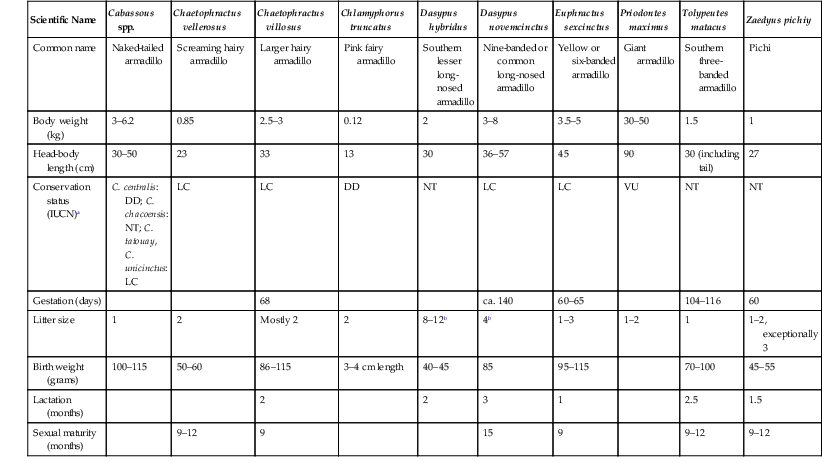
Scientific Name
Cabassous spp.
Chaetophractus vellerosus
Chaetophractus villosus
Chlamyphorus truncatus
Dasypus hybridus
Dasypus novemcinctus
Euphractus sexcinctus
Priodontes maximus
Tolypeutes matacus
Zaedyus pichiy
Common name
Naked-tailed armadillo
Screaming hairy armadillo
Larger hairy armadillo
Pink fairy armadillo
Southern lesser long-nosed armadillo
Nine-banded or common long-nosed armadillo
Yellow or six-banded armadillo
Giant armadillo
Southern three-banded armadillo
Pichi
Body weight (kg)
3–6.2
0.85
2.5–3
0.12
2
3–8
3.5–5
30–50
1.5
1
Head-body length (cm)
30–50
23
33
13
30
36–57
45
90
30 (including tail)
27
Conservation status (IUCN)a
C. centralis: DD; C. chacoensis: NT; C. tatouay, C. unicinctus: LC
LC
LC
DD
NT
LC
LC
VU
NT
NT
Gestation (days)
68
ca. 140
60–65
104–116
60
Litter size
1
2
Mostly 2
2
8–12b
4b
1–3
1–2
1
1–2, exceptionally 3
Birth weight (grams)
100–115
50–60
86–115
3–4 cm length
40–45
85
95–115
70–100
45–55
Lactation (months)
2
2
3
1
2.5
1.5
Sexual maturity (months)
9–12
9
15
9
9–12
9–12
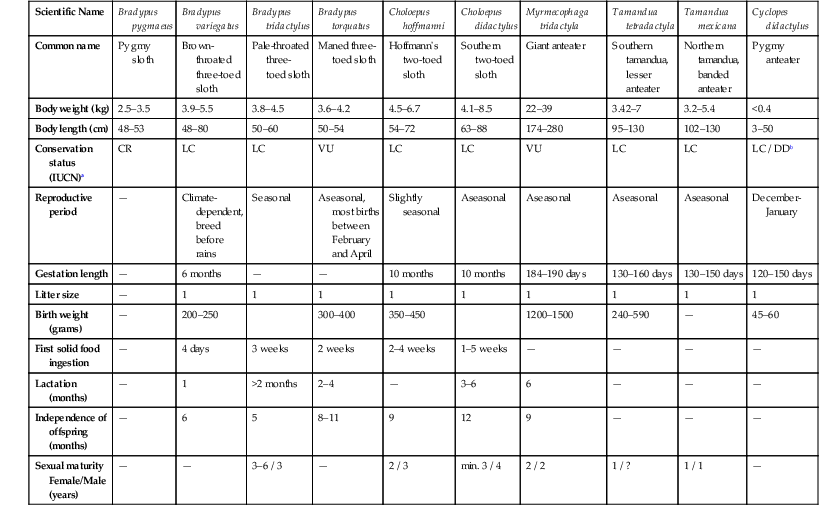
Scientific Name
Bradypus pygmaeus
Bradypus variegatus
Bradypus tridactylus
Bradypus torquatus
Choloepus hoffmanni
Choloepus didactylus
Myrmecophaga tridactyla
Tamandua tetradactyla
Tamandua mexicana
Cyclopes didactylus
Common name
Pygmy sloth
Brown-throated three-toed sloth
Pale-throated three-toed sloth
Maned three-toed sloth
Hoffmann’s two-toed sloth
Southern two-toed sloth
Giant anteater
Southern tamandua, lesser anteater
Northern tamandua, banded anteater
Pygmy anteater
Body weight (kg)
2.5–3.5
3.9–5.5
3.8–4.5
3.6–4.2
4.5–6.7
4.1–8.5
22–39
3.42–7
3.2–5.4
<0.4
Body length (cm)
48–53
48–80
50–60
50–54
54–72
63–88
174–280
95–130
102–130
3–50
Conservation status (IUCN)a
CR
LC
LC
VU
LC
LC
VU
LC
LC
LC / DDb
Reproductive period
—
Climate-dependent, breed before rains
Seasonal
Aseasonal, most births between February and April
Slightly seasonal
Aseasonal
Aseasonal
Aseasonal
Aseasonal
December-January
Gestation length
—
6 months
—
—
10 months
10 months
184–190 days
130–160 days
130–150 days
120–150 days
Litter size
—
1
1
1
1
1
1
1
1
1
Birth weight (grams)
—
200–250
300–400
350–450
1200–1500
240–590
—
45–60
First solid food ingestion
—
4 days
3 weeks
2 weeks
2–4 weeks
1–5 weeks
—
—
—
—
Lactation (months)
—
1
>2 months
2–4
—
3–6
6
—
—
—
Independence of offspring (months)
—
6
5
8–11
9
12
9
—
—
—
Sexual maturity Female/Male (years)
—
—
3–6 / 3
—
2 / 3
min. 3 / 4
2 / 2
1 / ?
1 / 1
—
Unique Anatomy and Physiology
Special Housing Requirements
Feeding
Restraint and Handling
Physical Restraint
Chemical Restraint
Diagnostics
Xenarthra
Chapter 39