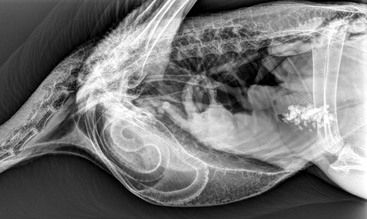
Robert A. MacLean, Hugues Beaufrère The order Gruiformes has historically been problematic to classify and does not seem to be a monophyletic group as at least four different clades exist in it. It includes the families Gruidae (cranes, 15 species), Psophiidae (trumpeters, 3 species), Rallidae (rails, crakes, soras, gallinules, swamphens, takahes, moorhens, and coots, 138 species), Sarothruridae (flufftails, 7 species), Heliornithidae (sungrebes, and finfoots, 3 species), and Aramidae (limpkins, 1 species). The orders Otididiformes (bustards, 26 species), Eurypygiformes (sunbitterns and Kagus, 2 species), Cariamiformes (seriemas, 2 species), and Mesitornithiformes (mesites, 2 species, and monias, 1 species) are traditionally included in the Gruiformes but are now largely separated because of morphologic and phylogenetic distinctions.12 This chapter presents information derived primarily from experience with managing cranes, as more published data are available, with some additional information provided for bustards, coots, and rails. Sustained efforts are ongoing to maintain, breed, and rear many species of Gruiformes for captive management and for reintroduction efforts. Much of our scientific knowledge, captive management techniques, and veterinary experience derive from these programs. According to the International Union for Conservation of Nature (IUCN) and Convention on International Trade in Endangered Species (CITES) (websites accessed November 16, 2012), of the 15 extant species of cranes, 14 species and subspecies are vulnerable or endangered; in bustards, 6 species are vulnerable or endangered; and in rails, 35 species are vulnerable or endangered, and one, the Guam rail (Gallirallus owstoni), is extinct in the wild (reintroduction efforts are ongoing). Cranes are found throughout the world except in the neotropics and the Antarctic. They differ from herons and egrets in that many crane species have bright-red thick skin covering parts of the head and neck and have perforate nares. Cranes are long-lived, with a captive lifespan that may reach 50 years. Crane adults, with the exception of the African crowned cranes (Balearica spp.), have a long, convoluted trachea that is enclosed within the bones of the keel, which is thought to be an adaptation for producing their loud bugling call (Figure 19-1). Cranes share this tracheal characteristic only with swans (Cygninae). An extremely long and coiled trachea is also a characteristic of trumpeters (Psophiidae), but the tracheal elongation is subcutaneous. Cranes may sustain leg and wing injuries during capture, and they are prone to skin lacerations from their sharp toenails when mishandled. The recommended method of capture is to gently herd or push the bird into a corner or other confined space using raised arms, brooms, or both. The folded flight feathers (or bustle) may be grasped by the handler with one hand, maintaining the wings in a closed position, and the free arm may grasp around the body and wings while directing the head and neck behind them. The hand holding the bustle may then be used to restrain the legs at the hock using one or two fingers between the hocks. The bird is picked up, and the legs are extended parallel to the ground (Figure 19-2). For the bird that insists on facing the handler prior to capture, handlers should gently grasp the neck and direct the head behind them to initiate the grab.10 Safety eyewear is highly recommended. Caution is advised, as aggressive cranes may cause serious injury, including punctures and lacerations. Other recommendations for restraint and handling have been outlined elsewhere.63 Kori bustards (Ardeotis kori) are powerful birds with very strong legs capable of leaping high into the air and upon restraint may cause substantial injury to themselves and to the handlers. Experienced handlers are strongly recommended for bustards (see the AZA Gruiformes TAG 2009 Kori Bustard [Ardeotis kori] Care Manual, Association of Zoos and Aquariums). Chemical restraint is appropriate for extensive handling or prolonged procedures to reduce stress-related complications, including hyperthermia and capture myopathy. Inhalant anesthesia, using isoflurane or sevoflurane administered via a mask; intubation, using an uncuffed endotracheal tube; and intermittent positive pressure ventilation (IPPV) are the preferred techniques for chemical restraint in captive Gruiformes. An appropriate facemask to accommodate the long beak of the crane may be made using a 60-milliliter (mL) plastic syringe case. Appropriate assessment of vital signs is recommended and should include monitoring of heart rate via Doppler blood flow monitor, end-tidal carbon dioxide (CO2) and respiratory rate with the use of a capnograph, and core body temperature (published recommended values for cranes are not available but seem comparable with those for birds of similar size). The minimum alveolar concentration (MAC) in Mississippi sandhill cranes is 1.34% ± 0.14%, and cranes are susceptible to dose-dependent, isoflurane-induced respiratory depression and hypotension, particularly under spontaneous ventilation.40 Therefore, it is recommended that a multimodal approach to anesthesia and controlled ventilation be used in these species. In addition, cranes have larger tracheal dead space compared with most birds, and inadequate ventilation and hypercapnia are frequently accompanied by bradycardia. Moreover, the interval between respiratory arrest and cardiac arrest may be very short in birds, leaving little time to respond. To reduce the chance for injury during recovery, the bird should be manually restrained in a quiet place, hooded or in low light, until it is likely to stand on its own when released. Mild to moderate sedation with midazolam (0.5 to 1.5 milligrams per kilogram [mg/kg]) and butorphanol (0.5 to 1.5 mg/kg) intramuscularly (IM) may be adequate for minor procedures and more prolonged handling such as bandaging or diagnostic imaging in cranes. Diazepam (0.5 to 1.0 mg/kg, orally [PO]) lasts 4 to 6 hours and is useful for tranquilization for procedures such as shipping agitated birds. Sedatives may be reversed for a rapid recovery, if necessary or desired. Local anesthetics (lidocaine 0.5 milliliters [mL], xylocaine 0.5 mL, or bupivacaine up to 2 mg/kg) may be infiltrated into areas for minor procedures such as wound suturing or implanting subcutaneous radio transmitters.51 For field work, cranes have been successfully anesthetized with ketamine and xylazine (10–15 mg/kg ketamine, 1 mg/kg xylazine, IM), ketamine and diazepam (10–15 mg ketamine, 0.2–0.5 mg/kg diazepam, IM) combinations, or with propofol (PropoFlo Injectable, Abbott Laboratories, North Chicago, IL) as a bolus intravenously (IV) or as a continuous rate infusion (CRI).51 A mixture of alphaxolone and alphadolone has also been used successfully in cranes for short procedures (6.5–7.0 mg/ kg, IV).2 Alpha-chloralose (0.39–0.48 gram per cup [g/cup], or 280 mL, of corn) has been used to capture wild cranes and cranes that have escaped from zoos or private collections; however, the ingested dosage is variable, and an increased risk of capture myopathy exists.29,49 Blood may be collected from the right jugular vein or from the medial metatarsal vein. Although very rare, jugular lacerations resulting in death have occurred in a struggling crane, so appropriate caution is advised. The basilic or ulnar veins may also be used but are not recommended in alert cranes. Normal blood values of select cranes and bustards are listed in Table 19-1.10,16,24,28,32,35,54 Hemolysis induced by EDTA (ethylenediaminetetraacetic acid) has been reported in crowned cranes, so heparin is recommended in these species.9 The looping trachea present in other crane species may lead to the buildup of mucus or fluids at certain points and result in severe dyspnea; because of this unique anatomy, any tracheal flushes should be used with extreme caution.51 TABLE 19-1 Hematologic and Serum Biochemical Reference (Mean [Range] or Mean +/− SD) Values for Select Captive Gruiformes a Reported as mean ± standard error of mean (Note: sem = sd/√n). * Calculated from reported mean absolute values. ALP, Alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; Chol, cholesterol; g/dL, gram per deciliter; Hb, hemoglobin; K, potassium; LDH, lactate dehydrogenase; mEq/L, milliequivalent per liter; mg/dL, milligram per deciliter; µL, microliter; P, phosphorus; PCV, packed cell volume; RBC, red blood cells; TP, total protein; Units/L, units per liter; WBC, white blood cells. Most medical treatments, procedures, and therapeutic regimens appropriate for use in other avian species should be generally applicable to Gruiformes and Otididae. Drug selection, dosage, route and frequency of administration may vary. Refer to Tables 19-24,7,10,15,39,47,48 and 19-37,8,10,48 for antimicrobials and anthelmintics commonly used in cranes. TABLE 19-2 Antimicrobial Agents Commonly Used in Cranes * Because these drugs have the potential to cause necrosis when administered intramuscularly, oral administration is preferable or it should be diluted in subcutaneous fluids. † According to pharmacokinetics in parrots, hawks, and owls. IM, Intramuscularly; IU/kg, international unit per kilogram; IV, intravenously; mg/kg, milligram per kilogram; mL, milliliter; PO, by mouth; PK, pharmacokinetic study; SQ, subcutaneously. TABLE 19-3 Antiparasitic Agents Commonly Used in Cranes g/ton, Gram per ton; IM, intramuscularly; mg/kg, milligram per kilogram; mL/L, milliliter per liter; PO, by mouth; ppm, parts per million; prn, pro re nata (as circumstances require); SQ, subcutaneously; tsp/gal, teaspoon per gallon.
Gruiformes (Cranes, Limpkins, Rails, Gallinules, Coots, Bustards)
Biology
Restraint and Handling
Diagnostic Procedures
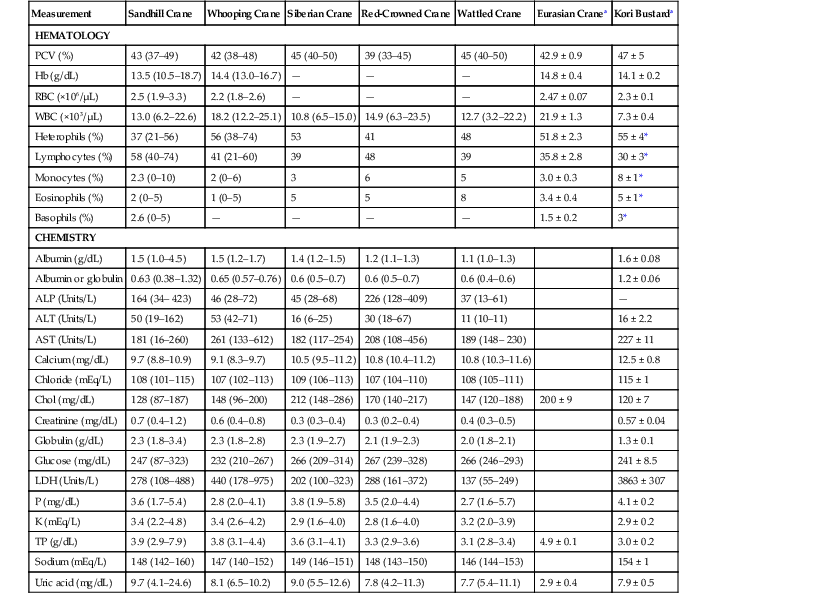
Measurement
Sandhill Crane
Whooping Crane
Siberian Crane
Red-Crowned Crane
Wattled Crane
Eurasian Cranea
Kori Bustarda
HEMATOLOGY
PCV (%)
43 (37–49)
42 (38–48)
45 (40–50)
39 (33–45)
45 (40–50)
42.9 ± 0.9
47 ± 5
Hb (g/dL)
13.5 (10.5–18.7)
14.4 (13.0–16.7)
—
—
—
14.8 ± 0.4
14.1 ± 0.2
RBC (×106/µL)
2.5 (1.9–3.3)
2.2 (1.8–2.6)
—
—
—
2.47 ± 0.07
2.3 ± 0.1
WBC (×103/µL)
13.0 (6.2–22.6)
18.2 (12.2–25.1)
10.8 (6.5–15.0)
14.9 (6.3–23.5)
12.7 (3.2–22.2)
21.9 ± 1.3
7.3 ± 0.4
Heterophils (%)
37 (21–56)
56 (38–74)
53
41
48
51.8 ± 2.3
55 ± 4*
Lymphocytes (%)
58 (40–74)
41 (21–60)
39
48
39
35.8 ± 2.8
30 ± 3*
Monocytes (%)
2.3 (0–10)
2 (0–6)
3
6
5
3.0 ± 0.3
8 ± 1*
Eosinophils (%)
2 (0–5)
1 (0–5)
5
5
8
3.4 ± 0.4
5 ± 1*
Basophils (%)
2.6 (0–5)
—
—
—
—
1.5 ± 0.2
3*
CHEMISTRY
Albumin (g/dL)
1.5 (1.0–4.5)
1.5 (1.2–1.7)
1.4 (1.2–1.5)
1.2 (1.1–1.3)
1.1 (1.0–1.3)
1.6 ± 0.08
Albumin or globulin
0.63 (0.38–1.32)
0.65 (0.57–0.76)
0.6 (0.5–0.7)
0.6 (0.5–0.7)
0.6 (0.4–0.6)
1.2 ± 0.06
ALP (Units/L)
164 (34– 423)
46 (28–72)
45 (28–68)
226 (128–409)
37 (13–61)
—
ALT (Units/L)
50 (19–162)
53 (42–71)
16 (6–25)
30 (18–67)
11 (10–11)
16 ± 2.2
AST (Units/L)
181 (16–260)
261 (133–612)
182 (117–254)
208 (108–456)
189 (148– 230)
227 ± 11
Calcium (mg/dL)
9.7 (8.8–10.9)
9.1 (8.3–9.7)
10.5 (9.5–11.2)
10.8 (10.4–11.2)
10.8 (10.3–11.6)
12.5 ± 0.8
Chloride (mEq/L)
108 (101–115)
107 (102–113)
109 (106–113)
107 (104–110)
108 (105–111)
115 ± 1
Chol (mg/dL)
128 (87–187)
148 (96–200)
212 (148–286)
170 (140–217)
147 (120–188)
200 ± 9
120 ± 7
Creatinine (mg/dL)
0.7 (0.4–1.2)
0.6 (0.4–0.8)
0.3 (0.3–0.4)
0.3 (0.2–0.4)
0.4 (0.3–0.5)
0.57 ± 0.04
Globulin (g/dL)
2.3 (1.8–3.4)
2.3 (1.8–2.8)
2.3 (1.9–2.7)
2.1 (1.9–2.3)
2.0 (1.8–2.1)
1.3 ± 0.1
Glucose (mg/dL)
247 (87–323)
232 (210–267)
266 (209–314)
267 (239–328)
266 (246–293)
241 ± 8.5
LDH (Units/L)
278 (108–488)
440 (178–975)
202 (100–323)
288 (161–372)
137 (55–249)
3863 ± 307
P (mg/dL)
3.6 (1.7–5.4)
2.8 (2.0–4.1)
3.8 (1.9–5.8)
3.5 (2.0–4.4)
2.7 (1.6–5.7)
4.1 ± 0.2
K (mEq/L)
3.4 (2.2–4.8)
3.4 (2.6–4.2)
2.9 (1.6–4.0)
2.8 (1.6–4.0)
3.2 (2.0–3.9)
2.9 ± 0.2
TP (g/dL)
3.9 (2.9–7.9)
3.8 (3.1–4.4)
3.6 (3.1–4.1)
3.3 (2.9–3.6)
3.1 (2.8–3.4)
4.9 ± 0.1
3.0 ± 0.2
Sodium (mEq/L)
148 (142–160)
147 (140–152)
149 (146–151)
148 (143–150)
146 (144–153)
154 ± 1
Uric acid (mg/dL)
9.7 (4.1–24.6)
8.1 (6.5–10.2)
9.0 (5.5–12.6)
7.8 (4.2–11.3)
7.7 (5.4–11.1)
2.9 ± 0.4
7.9 ± 0.5
Medical Treatments
Agent
Dosage
ANTIBIOTICS
Amikacin
10 mg/kg, IM, every 12 hours
Ampicillin
20 (to 100) mg/kg, IM, every 12 hours
Carbenicillin
100 mg/kg, IM or IV, every 12 hours
Cefazolin sodium
25–30 mg/kg, IM or IV, every 8 hours
Cefotaxime sodium
50–100 mg/kg, IM, every 8 hours
Cephalexin
35–50 mg/kg, PO, every 6 hours (PK)
Cephalothin
100 mg/kg, IM, SQ, every 6 hours (PK)
Chloramphenicol
100 mg/kg, SQ, every 8 hours
Enrofloxacin
8–15 mg/kg, PO or IM,* every 12 hours
Gentamicin
5 mg/kg, IM, every 8 hours (PK)
Piperacillin
100 mg/kg, IM, every 4–6 hours†
Trimethoprim–sulfamethoxazole
16–24 mg/kg (based on trimethoprim) PO every 12 hours
Tylosin
15 mg/kg, SQ, every 8 hours (PK)
ANTIMYCOTICS
Amphotericin B
5–10 mg/15 mL water; nebulize 20 minutes every 8 hours
Clotrimazole
30 mg (3 mL) in glycerol glycolate; nebulize 20 minutes every 8 hours
Flucytosine
100 mg/kg, PO, every 8–12 hours
Itraconazole
5–10 mg/kg, PO, every 12 hours
Nystatin
300,000 IU/kg, PO, every 12 hours
Agent
Dosage
Comments
Albendazole
20 mg/kg, PO; repeat in 7 days
Trematodes (some)
Amprolium
0.0125 mg/kg of feed, continuous 0.025 mg/kg of feed for 14 days 0.006% in drinking water, continuous
Coccidiosis: Prophylactic Coccidiosis: Therapeutic Coccidiosis
Carbaryl powder (5%)
Topical, weekly or biweekly, prn
Ectoparasites; use sparingly
Fenbendazole
50–100 mg/kg, PO; repeat in 14 days, prn
Intestinal strongyles, ascarids
50 mg/kg, PO, for 5 days; repeat in 14 days
Gapeworms and capillarids
Ivermectin
0.2 mg/kg, SQ; repeat in 14 days
Nematodes and mites
Levamisole
40 mg/kg (25 mg/kg in chicks), PO; repeat in 14 days, prn
Intestinal strongyles, ascarids, and capillarids
Monensin
90 g/ton of feed, 99 ppm (continuous or seasonally)
Coccidiosis, recommended for the prevention of visceral coccidiosis
Ormetoprim-sulfadimethoxine
0.015% ormetoprim and 0.026% sulfadimethoxine in food continuously for 3 weeks
Coccidiosis
Praziquantel
6 mg/kg, PO or IM; repeat in 10–14 days
Cestodes and trematodes
Pyrantel pamoate
4.5 mg/kg, PO; repeat in 10–14 days
Intestinal nematodes
Pyrethrin powder (0.10%)
Topical; repeat in 7–14 days, prn
Ectoparasites; apply lightly
Pyrethrin 0.03%, piperonyl butoxide 0.30% spray
Topical; repeat in 3 to 7days, prn
Ectoparasites; apply lightly
Sulfachlorpyridazine
1 tsp/gal (1.3 mL/L) drinking water for 7–14 days
Coccidiosis
Sulfadimethoxine
50 mg/kg, PO, every 24 hours for 14 days
Coccidiosis
Thiabendazole
100 mg/kg, PO; repeat in 7–14 days
Intestinal strongyles and ascarids
Trimethoprim-sulfamethoxazole
16–24 mg/kg (based on trimethoprim), PO, every 12–24 hours
Coccidiosis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Gruiformes (Cranes, Limpkins, Rails, Gallinules, Coots, Bustards)
Chapter 19