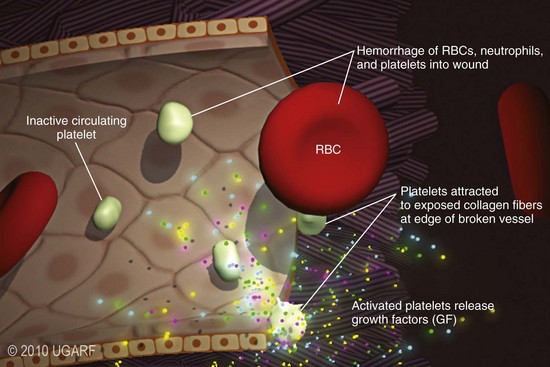
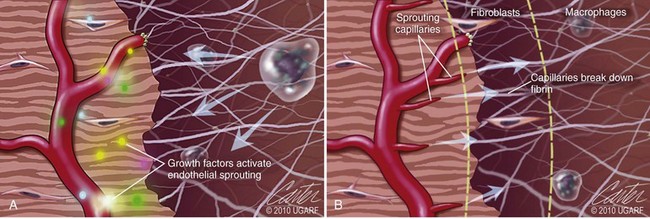
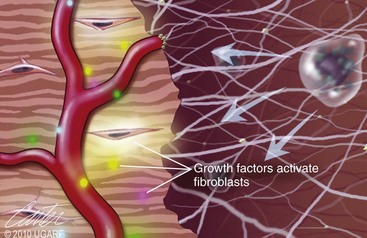
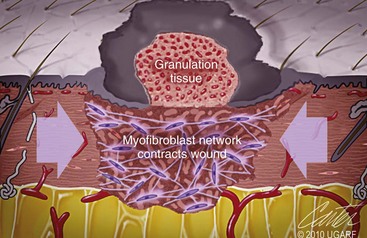
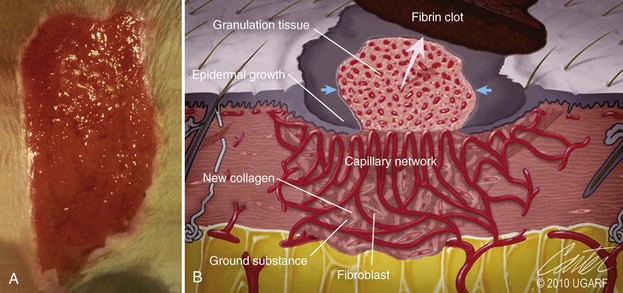
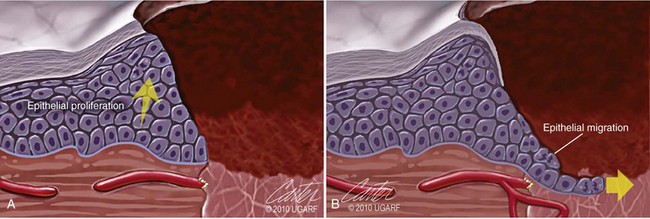
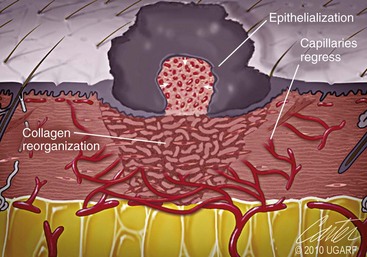
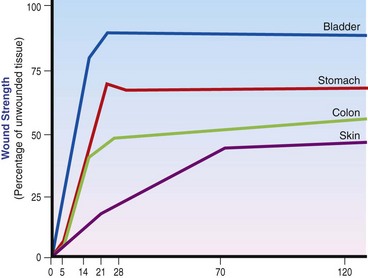
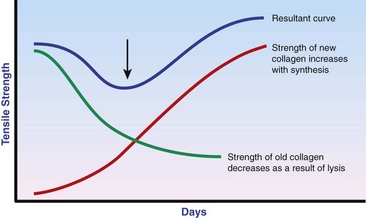
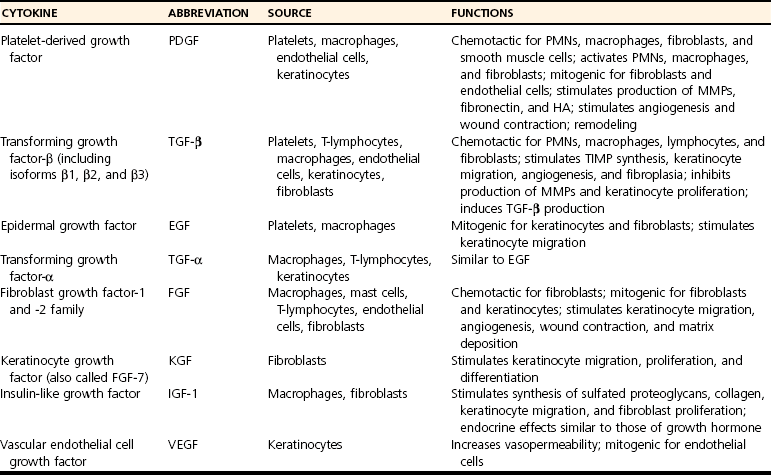
Chapter 9 Wound repair is the effort of injured tissues to restore normal function and structural integrity after injury.37 Wound healing is a complex biologic process that is described as discrete phases to facilitate understanding of the overall process. However, these phases actually overlap with respect to duration, as well as the cell type and cellular signaling involved. It is important that we understand that within any single wound more than one phase of wound healing may, and usually will, occur simultaneously. As our knowledge of cellular interactions and inflammatory mediators grows, so does our understanding of the truly complex nature of wound healing. The inflammatory phase of wound healing begins at the time of wounding. Tissue disruption initiates hemostasis and inflammation. As the vascular endothelium in the wound is disrupted, it produces endothelin, which, in combination with other mediators such as epinephrine, norepinephrine, and prostaglandins, initiates contraction of smooth muscle within the vessel walls, resulting in vasoconstriction.102 The coagulation cascade (see Chapter 7) is initiated, and thrombin is formed. Thrombin serves as a catalyst for fibrinogen conversion to fibrin and contributes to platelet activation.40 Activated platelets adhere to exposed subendothelial collagen of the disrupted vessel wall, aggregate together, and degranulate (Figure 9-1). Release of platelet alpha granules and their contents, including platelet-derived growth factor (PDGF), transforming growth factor-β (TGF-β), epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), and fibroblast growth factor (FGF), serves to attract to the local environment other cells required for wound healing (Table 9-1). PDGF is an important growth factor involved in attracting neutrophils to the wound site to remove bacteria. Thromboxane A2 and serotonin, produced by activated platelets, aid in mediation of initial vasoconstriction. Later, vasodilation and increased vascular permeability occur in response to the presence of leukotrienes, prostaglandins, histamine, and kinins. Vasodilation results in increased blood flow to the wound bed and extravasation of fluid, creating the classic signs of inflammation—heat, redness and swelling. Table • 9-1 Growth Factors in the Wound Healing Process and Cells That Produce Them Modified from Schwartz SI, editor: Principles of surgery, ed 7, New York, 1999, McGraw-Hill. Leukocyte migration from dilated vessels into the wound bed occurs in two phases: neutrophil migration and monocyte migration. Large numbers of neutrophils are activated through stimulation by multiple factors within the wound, including TGF-β, prostaglandins, tumor necrosis factor-α (TNF-α), interleukin (IL)-1, complement, and bacterial products.68 Neutrophils arrive in the wound within 24 to 48 hours of wounding. Neutrophils have many functions within the wound, including the killing of bacteria through release of reactive oxygen species, breakdown of extracellular matrix via release of proteolytic enzymes, phagocytosis of degraded bacteria and matrix debris, and release of additional cytokines that prolong the inflammatory phase.9,31 Neutrophil killing of bacteria is dependent upon a high partial pressure of oxygen within the wound. In vitro oxygen partial pressure below 40 mm Hg is associated with an inability of neutrophils to kill bacteria. After phagocytosis of a pathogen, primary oxidase (nicotinamide adenine dinucleotide phosphate [NADPH] oxidase) uses oxygen as the substrate to catalyze the formation of bactericidal superoxide. Superoxide initiates a series of cascades that produce other oxidants, hydrogen peroxide included, which increase bacterial killing capacity. The conversion of oxygen to superoxide is sensitive to the partial pressure, not the content or saturation, of oxygen in the tissue. For this reason, wound hypoxia impairs resistance to infection. TNF-α amplifies neutrophil chemotaxis and stimulates macrophage, keratinocyte, and fibroblast expression of growth factors needed in angiogenesis and collagen synthesis.53 Activated inflammatory cells consume oxygen at high rates, and when this process is combined with an impaired blood supply to the wound, local hypoxia develops.109 Lactate, produced within the wound secondary to hypoxia, stimulates collagen secretion and angiogenesis.61,64 Monocytes migrate through the vessel walls and, with the influence of TGF-β, mature to macrophages. Although neutrophils and monocytes both work to aid in debridement of the wound, the macrophage is essential for further secretion of signaling molecules, which, in turn, facilitate recruitment of the other cell types necessary for wound repair. Macrophages release cytokines, IL-1, IL-6, IL-8, TNF-α, and growth factors such as FGF, EGF, TGF-β, and PDGF. Approximately 48 to 96 hours after wounding, the macrophage has become the primary leukocyte in the wound.83 At this point, most neutrophils have been phagocytized by macrophages or have undergone apoptosis.36 Macrophages continue phagocytosis of debris, as well as secretion of proteases and removal of bacteria. Macrophages within the wound also release matrix metalloproteinases (MMP-1, MMP-2, MMP-3, and MMP-9) that degrade the extracellular matrix. The degradation of extracellular matrix facilitates movement of cells through the tissues. This phase of wound healing is characterized clinically by erythema and edema of the wound edges. Following debridement by macrophages, the wound enters a repair or proliferation phase. Following wound debridement, wound healing enters a constructive or repair phase that occurs from approximately day 4 through day 12.102 The goals of this phase include achieving permanent closure of the wound and replacing lost tissue. The duration of this phase is dependent upon many variables, including wound size and location, as well as the age and health of the individual animal. Predominant cell types of the repair or proliferative phase of wound healing include fibroblasts, endothelial cells, and epithelial cells. During this period, capillary ingrowth, collagen production, wound contraction, and wound coverage take place. Capillary ingrowth, or angiogenesis, occurs as a result of capillary sprouting from existing vasculature surrounding the wound (Figure 9-2, A). This sprouting occurs in response to production of growth factors, including VEGF and basic fibroblast growth factor (bFGF), by cells within and around the wound bed. VEGF is secreted predominantly by keratinocytes on the wound edge and also by macrophages, fibroblasts, platelets, and other endothelial cells. Similar to the neutrophil respiratory burst, the capillary endothelial response to VEGF and other angiogenic factors is oxygen dependent and therefore is reliant on arterial partial pressure of oxygen.71 Newly sprouted capillaries invade the wound and can become visible within approximately 4 days post injury (Figure 9-2, B). The result is the development of a microvascular network within the wound that provides oxygen and nutrients to the cells and becomes an integral part of the developing granulation tissue bed.107 Fibroblasts migrate into the wound from surrounding tissue and proliferate in response to PDGF, TGF-β, and EGF from platelets and macrophages (Figure 9-3). In response to PDGF, fibroblasts begin to synthesize type III collagen, glycosaminoglycans, and fibronectin.90 Collagen peptides are modified after translation to form triple helices that are transported from the cell. This helical configuration provides strength to healing tissues. Collagen production and posttranslational modification are additionally dependent upon oxygen.109 TGF-β peaks 7 to 14 days after wounding and helps to direct extracellular matrix production and degradation. TGF-β increases synthesis of type I collagen, decreases production of matrix metalloproteinases MMPs, and increases production of inhibitors of metalloproteinase.42 Interferon-inducible protein (IP-10) indirectly limits fibroblast recruitment and decreases production of interferons and platelet factor (PF4); these events together have a negative mitogenic effect on fibroblasts.55 Fibroblasts that are anchored within the wound are transformed into myofibroblasts in response to TGF-β1. Myofibroblasts form focal adhesions that provide adequate mechanical leverage to cause wound contraction49 (Figure 9-4). Differentiation into myofibroblasts is associated with increased expression of α–smooth muscle actin isotype.49 These anchored myofibroblasts exhibit less proliferation than fibroblasts migrating into the wound from the periphery.27,33,93 Grossly, this phase of wound healing is characterized by the development of a granulation bed (Figure 9-5, A), which is composed of a capillary bed, fibroblasts, macrophages, and a ground substance of collagen, fibronectin, and hyaluronic acid (Figure 9-5, B). At the edges of the wound, in response to EGF and TGF-α produced by activated platelets and macrophages, epithelial cells begin to proliferate (Figure 9-6, A) and move into the wound to minimize fluid loss and bacterial invasion. Initially, epidermal thickening and elongation of basal cells occur at the wound margin. Changes in cell surface integrin expression, along with decreased adherence to the laminin of the basal lamina, facilitate the migration of epithelial cells into the wound (Figure 9-6, B). Epithelial cells and endothelial cells secrete matrix metalloproteinases that break down fibrin in the path of cell migration. Epithelial cell migration continues until contact inhibition by epithelial cells already present in adjacent areas is noted. Migration is halted, although cellular proliferation continues in an attempt to re-create normal epidermal thickness. The final phase of wound repair is dedicated to remodeling and strengthening of collagen and is clinically the most important phase of wound healing. Early in the repair phase, the matrix is thin and allows migration of cells to within the wound bed. Before the development of isometric tension (equal tension in all directions), remodeling of the matrix depends on cell migration throughout the matrix and proteolysis of matrix proteins.19 This temporary matrix, which originated as fibrin and fibronectin produced through the process of hemostasis, has progressed to glycosaminoglycans and proteoglycans produced by fibroblasts. Thirty percent of the collagen in granulation tissue is type III collagen, but it is progressively remodeled and replaced so that the final scar contains only 10% type III collagen.33 The greater concentration of type III collagen found in granulation tissue is associated with more heavily glycosylated and thinner collagen fibrils arranged in parallel orientation; therefore, this matrix is relatively weak. As the collagen composition changes, eventually approximating the ratio of type I to type III collagen in intact tissues, stiffness increases, and the matrix becomes more rigid (Figure 9-7). Fibroblast function within the wound changes in response to mechanical loading on the matrix as it matures.49 Fibroblasts are stimulated by TGF-β to differentiate into myofibroblasts only in a loaded or stiff matrix. Therefore, as tension on the wound edges decreases secondary to collagen deposition, myofibroblasts regress, and collagen synthesis by other fibroblasts is progressively decreased. When the mechanical load is unending, for example, in wounds that cross joint surfaces, fibroblasts persist and continue to produce collagen.19 This results in abnormal healing in the form of contracture and frequently precludes normal function. Collagen accumulation within the wound is the result of both an increased number of fibroblasts within the wound and an increase in collagen production per cell.28 Although net collagen synthesis is complete by 4 to 5 weeks post wounding, collagen maturation may continue for 12 to 18 months. Strength of the matrix progresses from 3% of intact tissue strength at 1 week to 30% of intact tissue strength at 3 weeks. Three months after wounding, collagen has been reabsorbed and redeposited along the stress lines of the tissue, and the wound has achieved maximum strength at 80% of its original strength.19,37 Although the phases of wound healing are similar for all tissues, significant differences have been noted between the classic description of wound healing as it relates to skin versus the healing of other tissues. Variations in tissue repair are noted in cell types required, cellular signaling proteins required, duration of the healing process, and ability to regain pre-wound strength (Figure 9-8). The wall of the gastrointestinal tract, with the exception of the esophagus and the distal rectum, is composed of four layers: mucosa, submucosa, muscularis propria, and serosa.103 The mucosa is further subdivided into three components: the epithelium, the lamina propria (which is itself composed of loose connective tissue, blood vessels, lymphatics, mesenchymal cells, and inflammatory cells), and the muscularis mucosa, which is a thin layer of smooth muscle. The submucosa, composed of types I (68%), II (20%), and V (12%) collagen, was shown by Halsted in 1887 to provide the gastrointestinal tract with most of its tensile strength. It is critical for surgeons to recognize the importance of this layer and to know that it must be included in gastrointestinal closure techniques.46,52,104 In addition to collagen, the submucosa contains blood, lymphatic vessels, and nerve fibers. The muscularis propria is composed of smooth muscle and collagen divided into two layers: an inner circular layer and an outer longitudinal layer. The outermost layer, the serosa, comprises a thin layer of connective tissue with lymphatics, blood vessels, and mesothelial cells interspersed throughout. In working toward healing within the gastrointestinal tract, it is important to consider the nature of the injury. Disruptions of the mucosa alone heal via epithelial cell migration and proliferation. An epithelial seal of the defect and the creation of a barrier to luminal contents may be achieved within 3 days if direct mucosal apposition is accomplished.45 In contrast, longer periods of time are required to gain a seal with inversion or eversion of the mucosa.35,45 Full-thickness wound healing in the gastrointestinal tract begins with an inflammatory phase similar to that of skin healing. The initial phase of hemostasis and vasoconstriction is followed by diapedesis of inflammatory cells, including neutrophils and macrophages, into the wound. During the first 24 hours, neutrophils are the predominant cell; macrophages become the predominant cell after 48 hours.103,117 As seen in wound healing of the skin, macrophages are a key source of growth factors needed for healing of the gastrointestinal tract. Edema of the subepithelial region of the mucosa and submucosa occurs during this phase and may persist for up to 2 weeks.67 This edema and subsequent swelling must be anticipated in the decision of how tightly sutures should be applied, because ischemia may occur if sutures are overtightened. Fibrin, which is formed rapidly at the outer serosal layer, is essential for achieving a watertight seal. During the proliferation phase, granulation tissue is formed at the site of the wound, and collagen undergoes both synthesis and lysis. Unlike in the skin, where collagen is synthesized exclusively by fibroblasts, in the gastrointestinal tract, fibroblasts and smooth muscle cells synthesize collagen, with smooth muscle cells contributing more to absolute collagen formation than fibroblasts (Table 9-2).117 Collagen composition in the gastrointestinal tract differs from that in skin; three types of collagen (I, III, and V) are present in the gastrointestinal tract, whereas only two types of collagen (I and III) are present in the skin. Collagen-synthesizing smooth muscle cells are present in both the muscularis mucosa layer and the muscularis propria. Studies in rats have shown that the strength of anastomoses throughout the gastrointestinal tract decreases significantly (esophageal anastomoses 37%, gastroduodenal 64%, small intestine 70%, and colon 72%) in the first 48 hours after surgery.58 Collagen breakdown secondary to collagenase activity within the wound occurs during this “lag phase” in the first 1 to 2 days of the healing process and results in net loss of strength of the anastomotic closure. The strength of the remaining collagen and its ability to hold suture during this time is critical to the success of the anastomosis. Table • 9-2 Comparison of Factors Involved in Healing of the Gastrointestinal Tract and Skin Intra-abdominal sepsis is associated with a generalized increased in collagenase activity and subsequently both greater loss and decreased production of collagen at the site of anastomosis.2,3 This net decrease in collagen at the anastomotic site increases the risk for failure. During the maturation phase of gastrointestinal healing, the collagen is remodeled, and the anastomotic site becomes stronger as the wall of the intestine becomes thinner (Figure 9-9).
Wound Healing
Phases of Wound Healing
Inflammation and Debridement
Proliferation
Remodeling and Maturation
Healing of Specific Tissues
Gastrointestinal Healing
GI TRACT
SKIN
Bacterial flora
Aerobic and anerobic
Aerobic, rarely causes a problem
Collagen subtypes
1, 3, 5
1, 3
Production
Smooth muscle cells and fibroblasts
Fibroblasts only
Collagenase activity
Increased days 0 to 3 causes decreased anastomotic strength
Not significant
Vascular perfusion
Significantly downregulated in states of shock and shunted to more critical organs
Relatively constant
Wound shear stress
Increased as the result of intraluminal transport
Minimal
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Wound Healing
Only gold members can continue reading. Log In or Register to continue