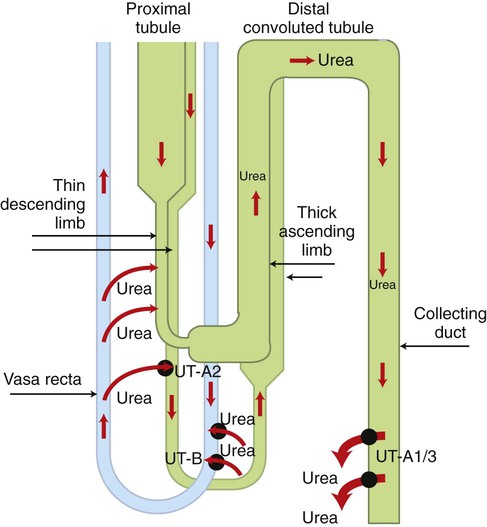
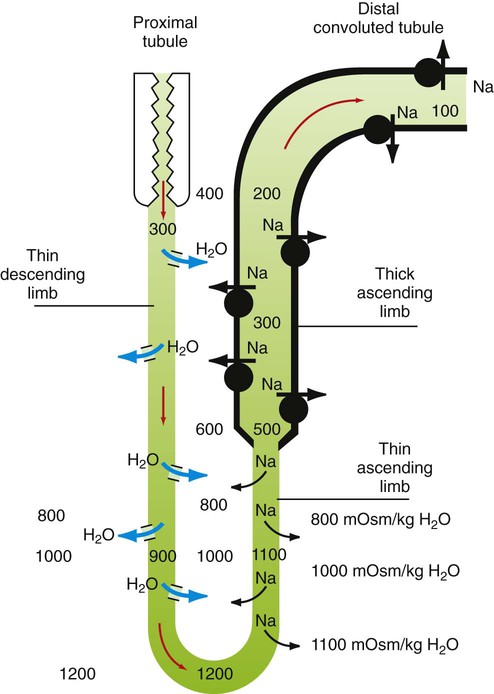
1. The kidney maintains water balance. 2. The proximal tubule reabsorbs more than 60% of filtered water. 3. The kidney can produce either concentrated or diluted urine. 4. A hypertonic medullary interstitium is needed to form concentrated urine. 5. Short-loop and long-loop nephrons have different roles in urine concentration. 6. Sodium chloride reabsorption by the medullary thick ascending limb generates medullary hypertonicity. 7. Urea reabsorption by the inner medullary collecting duct and urea recycling enhance medullary hypertonicity. 8. The countercurrent mechanism increases medullary interstitial osmolality with minimal energy expenditure. 9. Countercurrent exchange in the vasa recta removes water from the medullary interstitium without reducing medullary interstitial hypertonicity. 10. Active sodium chloride reabsorption in the thick ascending limb and the distal convoluted tubule dilutes the tubule fluid. 11. Antidiuretic hormone regulates collecting duct water permeability to determine the final urine osmolality. 12. Cells in the inner medulla adapt to interstitial hyperosmolality by accumulation of organic osmolytes. The anatomical arrangement of the renal tubules in the medulla is a crucial element of the urine-concentrating mechanism. The nephrons of the mammalian kidney are subdivided into superficial and juxtamedullary nephrons based on the location of their respective glomeruli (see Figure 41-1). The majority are superficial nephrons, which have short loops of Henle that extend only into the inner stripe of the outer medulla. These short-loop nephrons have a descending thin limb that parallels the thick ascending limb, but they do not have an ascending thin limb; the thin descending limb merges with the thick ascending limb near the hairpin turn (Figure 43-1). The inner medullary collecting duct (IMCD) also actively reabsorbs NaCl, but its more important contribution to the medullary hypertonicity is the reabsorption of urea (see Figure 43-1). Although the cortical and outer medullary collecting ducts are impermeable to urea, the terminal IMCD is highly permeable to urea via specific urea transporters (UT-A1, UT-A3). Thus, urea remains in the tubule fluid until it reaches the terminal IMCD deep in the medulla. Because urea reabsorption by the IMCD is enhanced by ADH, when conditions demand water conservation and ADH is released, urea reabsorption is enhanced and the osmotic gradient for water uptake increases. Because the thin limbs of Henle’s loop are permeable to urea, the high interstitial urea concentration drives urea into the thin limb luminal fluid. The tubule segments that intervene between the thin ascending limb and the terminal IMCD are impermeable to urea, thus urea reabsorbed from the terminal IMCD and taken up by the thin limbs is recycled back to the IMCD. In mammals this system of urea recycling enhances the efficiency of the urine-concentrating mechanism. In birds, however, urea is nearly absent in the medullary interstitium; urates do not contribute appreciably to osmotic pressure because they have low water solubility. Thus, medullary hypertonicity in birds appears to depend on single-solute (NaCl) recycling. The prevailing hypothesis for decades has been that a countercurrent mechanism in the thin limbs of Henle’s loop is responsible for the progressive amplification of the medullary hypertonicity initiated by the active reabsorption of salt by the thick ascending limb of Henle’s loop (Figure 43-2). This may be accomplished with minimal energy expenditure because of two characteristics: (1) the anatomical arrangement of the thin limbs of Henle’s loop and (2) the differential water and salt permeabilities of the descending and ascending thin limbs. Although recent data on the specific distribution of water and solute permeabilities and the complex anatomical associations of tubules and vessels in the medulla have raised doubts about the countercurrent multiplier hypothesis, the fundamentals of the concept remain the basis of understanding the mechanisms maintaining medullary hypertonicity.
Water Balance
Short-Loop and Long-Loop Nephrons Have Different Roles in Urine Concentration
Urea Reabsorption by the Inner Medullary Collecting Duct and Urea Recycling Enhance Medullary Hypertonicity
The Countercurrent Mechanism Increases Medullary Interstitial Osmolality with Minimal Energy Expenditure
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Water Balance
Only gold members can continue reading. Log In or Register to continue