Chapter 14 Vitreous
ANATOMY AND PHYSIOLOGY
Anatomy
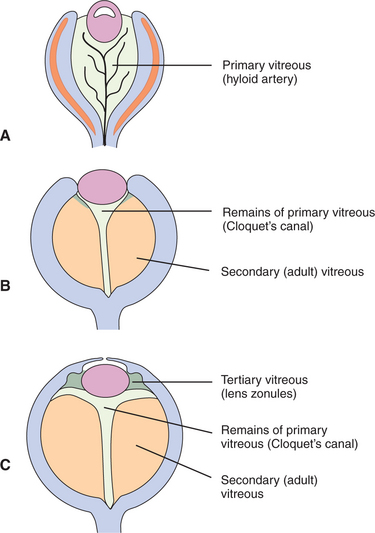
The vitreous is a transparent elastic hydrogel (Figure 14-1). It occupies about 80% of the volume of the eye (Figure 14-2). During embryonic development, primary, secondary, and tertiary vitreous are formed and laid down (Figure 14-3). Their genesis is described in detail in Chapter 2. Briefly, the primary vitreous is associated with the hyaloid vascular supply system, which nourishes the lens during development. The secondary vitreous is laid down around the primary vitreous and forms the definitive (adult) vitreous, whereas the tertiary vitreous contributes to the formation of the lens zonules.
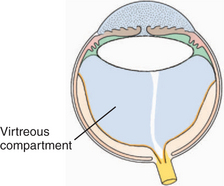
Figure 14-2 The vitreous body (shaded area) occupies the posterior compartment of the eye.
(Modified from Fine BS, Yanoff M [1979]: Ocular Histology. Harper & Row, New York.)
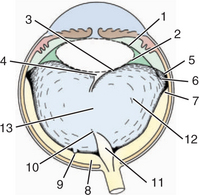
The vitreous body is divided into the following zones (Figure 14-4):
Composition
Vitreous is a complex gel with the following constituents:
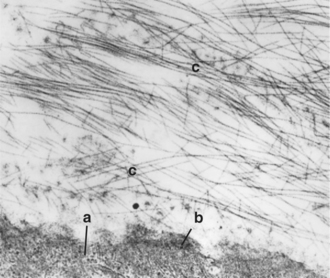
Collagen fibrils form a meshwork internal to the retina (the vitreous cortex) and intermingle with the fibers of the internal limiting membrane of the retina, thus forming a firm attachment between the vitreous cortex and the retina (Figure 14-5). Therefore anterior movement of the vitreous (such as occurs after lens luxation) may pull the retina off the retinal pigment epithelium (RPE) and cause traction retinal detachment. A potential space exists between the vitreous and the inner surface of the retina. Blood and exudates may accumulate in this space if the vitreous and retina separate, resulting in subhyaloid hemorrhage.
The collagen fibrils are also responsible for the numerous attachments of the vitreous to the adjacent structures—the posterior lens capsule, the ora ciliaris retinae (the vitreous base), and the optic nerve head (see Figure 14-4). Collagen fibrils are present in greater concentrations at the vitreous bases and around the optic disc, where attachment is the strongest.
PATHOLOGIC REACTIONS
Because of its simple structure and lack of vascular and lymphatic supply, the vitreous has a limited range of pathologic reactions, as follows:
Congenital and Developmental Abnormalities
Persistent Hyaloid Artery
The hyaloid artery is part of the embryonic vascular supply of the lens, which is described in Chapter 2. In most species, the hyaloid artery atrophies within a few weeks after birth (see Figure 14-3). An exception is ruminants, in which remains of the artery may be observed in a significant number of adults. However, persistent remnants of varying extent may be found in any species. The remnants of the artery origins on the surface of optic disc, which are surrounded by glial tissue, are called Bergmeister’s papilla (see Figure 14-4, area 11). These appear ophthalmoscopically, end-on, as red to white tufts originating from the optic disc and extending anteriorly a variable distance into the vitreous (see Figure 2-10, B, in Chapter 2). Similarly, at the distal end of the artery, remains of its attachment to the posterior lens capsule may be seen. The remains are known as Mittendorf’s dot (see Figure 14-4, area 4). It does not interfere with vision, except for rare occasions when it induces focal, posterior cataracts. A persistent artery, however, may extend from the disc all the way to the posterior lens. Persistence of the hyaloid artery may be hereditary in the Doberman pinscher and Sussex spaniel. In rats, the hyaloid artery may bleed into the vitreous during normal atrophy.
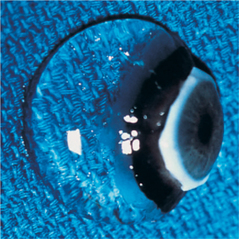
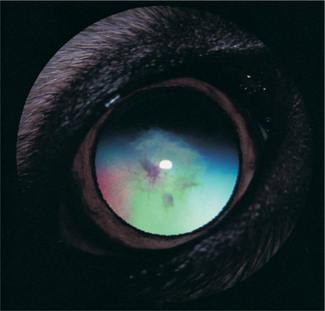
Persistent Hyperplastic Primary Vitreous
Clinically PHPV appears as a white or fibrovascular plaque in the posterior pupil near the posterior lens capsule and anterior vitreous (Figure 14-6). Vessel ingrowth and frank hemorrhage into the vitreous and lens substance, calcium deposits, posterior lenticonus, microphakia, lens coloboma, intralental pigmentation, progressive cataracts, and elongated ciliary processes may also be present. Surgery of cataracts associated with PHPV carries a guarded prognosis due to opacification of the posterior lens capsule, the possibility of a patent blood vessel, and the need to combine the surgery with anterior vitrectomy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree