Chapter 12 Vitreoretinal surgery
Types of retinal detachment 360
Types of vitreoretinal surgery 360
Clinical evaluation of retinal detachments 360
Instrumentation for vitreoretinal surgeries 361
Preoperative considerations for vitreal aspiration and removal 362
Preoperative considerations for retinal detachments 363
TYPES OF VITREORETINAL SURGERY 363
Adaptations for large animals and special species 369
Suprachoroidal cyclosporine implants for the treatment of equine recurrent uveitis 369
Pars plana (anterior) vitrectomy 370
Surgical anatomy
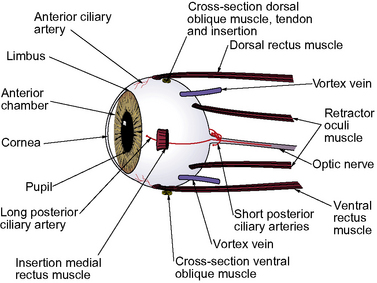
The surgical anatomy of the canine vitreoretinal surgeries includes the extraocular space, insertions of the rectus muscles, and the different landmarks of the external sclera (Fig. 12.1). The surgical approach to the anterior vitreous is usually through the pupil, posterior lens capsule (after extracapsular cataract surgery), and anterior vitreal membrane (after intracapsular cataract and lens removal). Vitreoretinal surgeries involve entry into the vitreal space through multiple small sclerotomies or surgical ports in the pars plana ciliaris. Incisions of the retina are avoided as the resultant hole may produce a rhegmatogenous retinal detachment. Full-thickness retinal holes, whether surgical or spontaneous, require laser, diathermy or cryotherapy to seal the area and stimulate formation of a strong chorioretinal scar. This scar prevents aqueous or vitreous from entering the subretinal space through the retinal break and causing a retinal detachment.
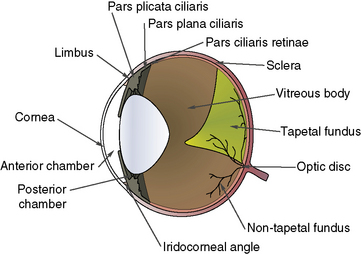
To perform vitreous paracentesis (hyalocentesis), the extent of the pars plana ciliaris or the flat posterior portion of the ciliary body is determined based on measurements posterior to the limbus (Fig. 12.2). The exact anterior and posterior borders of the pars plana ciliaris have been determined in the dog but not in the cat. The width of this tissue varies by quadrant, with the pars plana ciliaris in the lateral quadrant the longest. Hypodermic needle penetration of the ciliary body processes (pars plicata ciliaris) may result in considerable intraocular hemorrhage. Hypodermic needle penetration posterior of the pars plana ciliaris will produce retinal holes. Hence, access to the vitreal space without these serious complications necessitates the external penetration of the sclera and pars plana ciliaris, or the insertion of a hypodermic needle through the pupil after entry into the anterior chamber through a corneal or limbal incision into the anterior chamber.
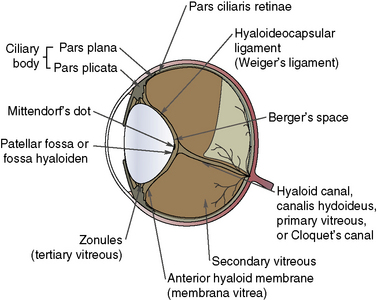
The anatomy of the vitreous is critical to vitreoretinal surgeries, as part of the surgical procedure will nearly always be within the vitreous space (Fig. 12.3). The vitreous humor is normally a clear gel that adjoins the entire retina, and this interface is termed the posterior vitreal membrane. This attachment to the retina appears relatively weak, except at the periphery of the retina (pars plana retinae) and the optic disk. The vitreous border continues anteriorly onto the pars plana ciliaris and, just before the origins and insertions of the zonules or suspensory ligaments between the ciliary body and lens equator, forms the posterior border of the posterior chamber.
The sclera constitutes the fibrous tunic for the entire posterior segment. There are several channels or emissaria where the full-thickness sclera is penetrated, and during vitreoretinal surgeries, these regions are avoided (see Fig. 12.1). These channels also represent weak areas of the sclera, and during glaucoma, focal enlargements or staphylomas may develop. At least four vortex veins exit the posterior segment, usually by quadrant and at the equator. These large veins provide the majority of venous drainage from the anterior and posterior segments of the eye and should be avoided. At the 3 and 9 o’clock positions within the external sclera are the long posterior ciliary arteries and veins that are branches of the external ophthalmic artery and vein. These vessels are avoided as they provide the majority of the blood supply to the anterior segment of the eye. Numerous anterior ciliary arteries and veins penetrate the anterior sclera at the insertions of the different rectus muscles. These blood vessels eventually anastomose with other intraocular vessels. Hence, transection of the rectus muscle insertions can decrease the blood supply to the anterior segment of the eye.
Surgical pathophysiology
Types of vitreoretinal surgery
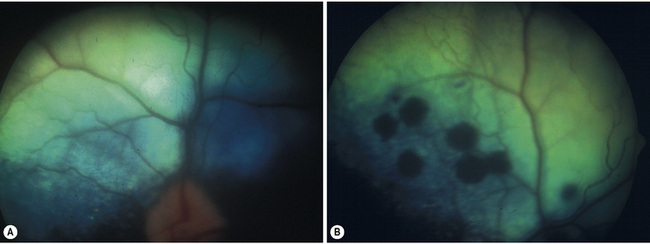
Non-rhegmatogenous retinal detachments are usually treated medically. If possible, the inciting cause is identified and treated. For instance, if systemic hypertension has resulted in the retinal detachment, systemic antihypertensive therapy is administered (Fig. 12.4). If the retinal detachment has resulted from a chorioretinitis, the appropriate therapy is administered. Retinal detachments are also treated symptomatically. Systemic drugs, such as corticosteroids and diuretics, may hasten the reabsorption of the subretinal fluids, and reapposition of the neurosensory retinal layers and the retinal pigment epithelium.
Clinical evaluation of retinal detachments
Once the fundoscopic examination has been performed and any retinal breaks identified and localized, retinal surgery may be indicated. The general principles for the surgical treatment of rhegmatogenous retinal detachments are summarized in Box 12.1, part A. Once the retinal traction is removed and the retinal breaks are sealed, normal physiologic function of the retina may resume with the pigmented epithelium to evacuate the subretinal fluids, and eliminate any space between the neurosensory retina and the retinal pigment epithelial layers.
Box 12.1 General principles for the treatment of rhegmatogenous retinal detachments
Part A Principles
1. Closure of retinal break: By diathermy, cryotherapy, or laser.
2. Collapse of the space between the separated retinal layers:
3. Excision of vitreal and/or inflammatory traction bands that appear to pull on the retina.
4. Occasional temporary intravitreal injections of silicone oil and/or perfluorocarbon gases to push and flatten (tamponade) the area of retinal detachment.
5. Development of focal chorioretinitis that eventually resolves to form scar tissue that adheres to the retinal layers within the detached area and, most importantly, around the retinal break.
Part B Specialized instruments for pars plana retinal detachment surgery
20 g microvitreoretinal (MVR) blades
20 g end gripping microforceps
20 g DeJuan intraocular forceps
20 g horizontal and vertical forceps
Modified from Smith PJ 1999 Surgery of the canine posterior segment. In: Gelatt KN (ed.) Veterinary Ophthalmology, 3rd edn. Lippincott, Williams and Wilkins, Baltimore, p 935–980.
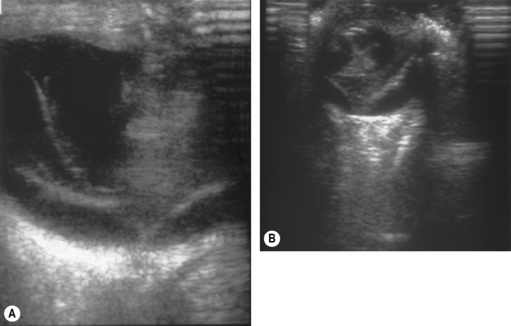
Additional invaluable diagnostic procedures for small animals are electroretinography and ultrasonography. Electroretinography may provide information as to the viability of the retina, and for detecting retinal detachments. Ultrasonography is used particularly to evaluate the posterior segment in which visualization is incomplete because of a small pupil, or lens or vitreal opacities (Fig. 12.5).
Instrumentation for vitreoretinal surgeries
Surgical instruments
Basic ophthalmic instruments are necessary to perform the surgical approach for retinal detachments. These instruments are necessary to perform the conjunctival (peritomy) and periocular surgery to isolate and sometimes transect the extraocular muscle insertions. The sclerotomies or small 20 g incisions through the sclera and pars plana of the ciliary body require a basic set of 20 g diameter instrumentation (Box 12.1, part B). These 20 g instruments can be inserted and withdrawn from these ports without causing damage. In addition, several of the instruments are essential. Posterior vitreous cutter, light source, IOP control, and wet-field cautery are necessary. The Machemer lens rests on the cornea and provides irrigation to the cornea, and a wide field of vision and magnification. To inject silicone oil, either a large bore high viscosity cannula with a Luer-lock syringe for manual injection or a special syringe pump and high-viscosity injection, are necessary. A nitrous oxide cryounit with a retinal probe and/or a diode laser with endolaser and indirect delivery modes are essential for retinopexy.
Preoperative considerations for retinal detachments
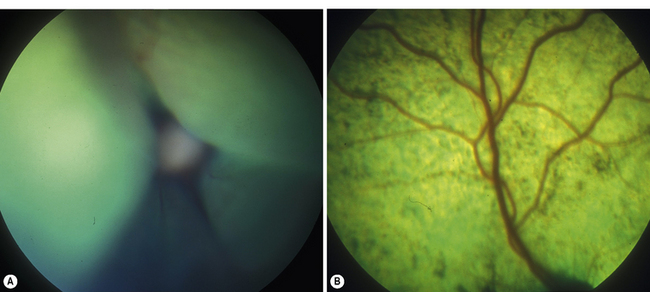
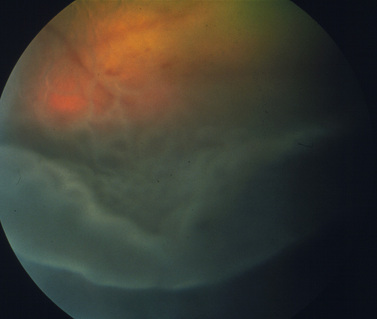
Surgical treatment of retinal detachment has been reported in dogs with serous retinal detachments secondary to optic disk pits or idiopathic (Fig. 12.6), and for rhegmatogenous retinal detachments associated with retinal holes or tears that developed after cataract surgery. As indicated in an earlier section, exudative retinal detachments associated with posterior segment inflammations, systemic hypertension, and other causes are treated by therapy for the specific systemic disorder, and systemic corticosteroids and diuretics to attempt to remove the subretinal fluids before the retinal degeneration becomes advanced. Unfortunately, most retinal detachments are presented late in small animals, and often entire retinas are detached in both eyes. Retinal detachments that develop after lens and cataract removal are often detected earlier during the periodic postoperative examinations (Fig. 12.7).
Surgery of the vitreous
Modern vitreous surgery techniques have been developed in humans since about 1965. With the demonstration that the excision of vitreous in humans did not result in the detachment of the retina and loss of vision, specialized instrumentation and techniques have been developed in humans to perform vitreoretinal surgeries through small incisions in the pars plana of the ciliary body. Anterior vitrectomy involves the excision of vitreous herniated or protruding through the pupil, as well as pupillary opacities that develop postoperatively after cataract surgeries in small animals. These pupillary opacities usually consist of anterior and posterior lens capsules, organized anterior vitreous and inflammatory fibropupillary membranes. In the former case, the herniated organized anterior vitreous is excised using cellulose sponges or forceps, and vitreous scissors utilizing an anterior vitrector (either portable or attached to a cataract surgical unit). For the postoperative pupillary opacities that develop in dogs and, to a lesser extent, in cats, the anterior vitreous and tough fibrotic membranes require excision by sharp scissors and forceps (anterior vitrectomy and pupillary membranectomy). Depending on its composition, the anterior vitreous may be excised (formed vitreous) or aspirated (liquid vitreous). Sometimes the irregular and often miotic pupil requires coreoplasty (see Chapter 9) to not only excise the opacities within the pupil but also enlarge the pupil to enhance vision.
Hyalocentesis/vitreous paracentesis
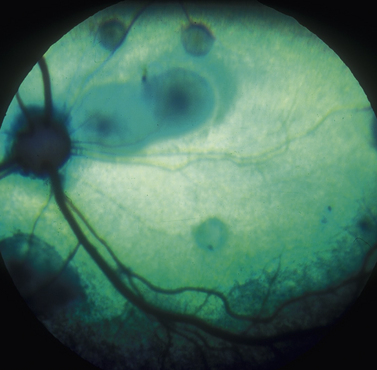
In vitreal paracentesis or hyalocentesis a small quantity of liquid vitreous is aspirated for analysis, usually cytology and bacterial/fungal culture. The formed gel vitreous cannot be aspirated; however, vitreous syneresis or liquefaction occurs with aging, cataract formation, intraocular inflammation, and glaucoma in small animals. As a result, some portion of the vitreous is liquefied, unless the cat or dog is less than 1 year old. Vitreal paracentesis is a most useful diagnostic technique for dogs and cats presented with bilateral exudative retinal detachments or endophthalmitis associated with Blastomyces dermatitidis, Histoplasma capsulatum, Cryptococcus neoformans, Coccidioides immitis, Geotrichum candidum, and Prototheca organisms (Fig. 12.8). Hyalocentesis may also be used to diagnose posterior segment neoplasia; however, this technique is not recommended for this purpose. Direct hypodermic needle contact of the neoplasm is usually associated with hemorrhage. However, if cellular material is present in the vitreous adjacent to the chorioretinal mass, aspiration of the vitreous in this area is reasonably safe.
Vitreous aspiration procedure
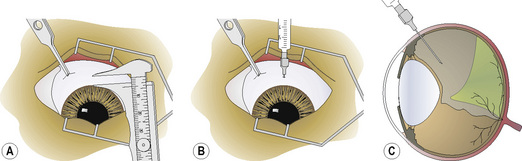
Hyalocentesis is performed after the onset of general anesthesia, clipping of the eyelid hair, and cleansing of the eyelid skin, conjunctival and corneal surfaces with 0.5% povidone–iodine solution. The pupil is dilated before the onset of general anesthesia. For convenience, the eyelids are retracted by speculum. The pupil and anterior vitreous are usually visible. The bulbar conjunctiva is grasped by thumb forceps with teeth several millimeters posterior to the dorsal limbus (Fig. 12.9a). By calipers the site to penetrate the bulbar conjunctiva is determined as 6–9 mm (dorsal) to 8–9 mm (lateral) posterior to the limbus. At this position, the 22–23 g hypodermic needle, aimed at the optic disk, should penetrate the sclera and pars plana ciliaris to enter the anterior vitreous (Fig. 12.9b).
Often once the hypodermic needle is in the anterior vitreous, it can be directly observed. If vitreal opacities or floaters are present, the hypodermic needle is carefully directed to these opacities (Fig. 12.9c). Aspiration of vitreous depends on it being liquefied. Formed vitreous or large opacities may plug the needle and require flushing to relieve the obstruction. The quantity of liquid vitreous aspirated is limited to 0.1–0.25 mL. If larger volumes are removed, an equal volume of saline or lactated Ringer’s solution is injected to immediately restore the lost vitreous volume and IOP.
Intravitreal injections
The same technique used for hyalocentesis can be used to deliver drugs directly into the vitreous space. As described in Chapter 10, intravitreal injections of gentamicin (10–25 mg), with and without 1 mg dexamethasone, have been used to destroy the ciliary body epithelia, and induce phthisis bulbi and ocular hypotony in advanced end-stage primary glaucomatous eyes.
Antibiotics and antifungal agents can be injected intravitreally for bacterial and fungal chorioretinitis, and endophthalmitis. Both volume and concentration of drugs injected intravitreally are limited. The retina and lens appear quite sensitive to drugs, and excess drug concentrations result in cataract formation and retinal degeneration. The doses of selected antibiotics and antifungal agents, based on those for humans, are summarized in Table 12.1.
Table 12.1 Doses for intravitreal antibiotics in the dog
| Antibiotic | Dose |
|---|---|
| Amikacin | 0.4 mg |
| Ampicillin | 5.0 mg |
| Cefazolin | 2.25 mg |
| Cephaloridine | 0.25 mg |
| Chloramphenicol | 2.0 mg |
| Gentamicin | 0.4 mg |
| Tobramycin | 0.4 mg |
| Vancomycin | 1.0 mg |
Transpupillary vitreal aspirations
The anterior chamber is maintained with viscoelastic agents.
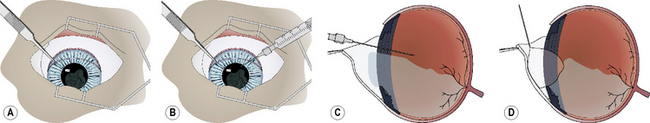
The formed vitreous may be indistinguishable from the aqueous humor or liquefied vitreous, unless some vitreal floaters (cells, fibrils, opacities) are present (Fig. 12.10a). A blunt 18–20 g hypodermic needle, attached to a 1–3 mL syringe, is carefully inserted through the pupil and the remaining posterior lens capsule and/or anterior hyaloid face about 5 mm into the most dorsal vitreal space. Insertion of the hypodermic needle through the external sclera and pars plana ciliaris, as used for hyalocentesis, is not recommended as it may collapse the globe and cause additional formed vitreous to appear in the pupil (Fig. 12.10b). Liquid vitreous is always above the formed or gel portion, and shifts to remain dorsal during the different positions of the eye. As a result, the hypodermic needle to aspirate liquid vitreous must be placed in the upper vitreal body. Liquefied vitreous is slowly aspirated, usually 0.1–0.5 mL. Often with removal of the liquid vitreous, the formed vitreous within the pupil and anterior chamber will partially or completely shift behind the pupil.
The iridal diaphragm may appear somewhat concave after successful vitreous aspiration, and restoration of all formed vitreous to behind the pupil (Fig. 12.10c). As the gel vitreous and solutions used to lavage the anterior chamber are indistinguishable, a small air bubble may be injected into the anterior chamber to assist in detection of any residual gel vitreous (Fig. 12.10d). The air bubble should be freely maneuverable through the entire anterior chamber when all gel vitreous has been removed or retracted through the pupil. If some gel vitreous remains within the anterior chamber, the air bubble movement is restricted and may outline the formed vitreous. No formed or gel vitreous should remain in the anterior chamber at the conclusion of this technique.
Anterior/partial vitrectomy in small animals
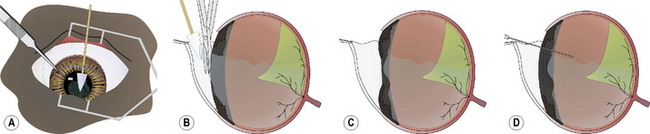
When the anterior vitrectomy is performed immediately after lens or cataract removal, the majority of the procedure is performed within the anterior chamber. The formed vitreous may be clear or contain suspended cells, fibrin, vitreous opacities (such as with hyalosis), and blood. In the anterior vitrectomy procedure for anterior chamber vitreal presentation, small cellulose surgical sponges are touched to the formed vitreous (Fig. 12.11a).The adherent gel vitreous is slowly retracted and carefully cut with sharp iris or vitreous scissors. The scissors are held parallel to the surface of the iris to minimize traction on the deeper vitreous and indirectly on the retina (Fig. 12.11b). All gel vitreous within the anterior chamber may be removed by this procedure. The iris surface should be slightly concave afterwards because of the loss of formed vitreous immediately posterior to it (Fig. 12.11c). Many phacoemulsification units also offer vitrectomy capacity that can be used to cut and aspirate the formed vitreous from the anterior chamber.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree