9 Vitamins
Introduction
The traditional view is that vitamins cannot be synthesized in sufficient quantities by more highly developed animals and therefore must be obtained from the diet. However, the validity of this statement depends on the vitamin and the species in question. As an example, vitamin D3 can be formed, under exposure to UV light, from a pre-cursor in the skin and is further activated in the liver and kidney (Schenk & Kolb 1982). Vitamin C can be synthesized in the liver of all mammals (Chattergee 1973) apart from guinea pigs, bats and one of the two major primate suborders (which includes human beings). Certain gut microbes are able to synthesize some vitamins (e.g., those of the B-complex) but the question remains, to what extent these vitamins can be absorbed from the gut and thus used by the host?
Certain provitamins exist (e.g., β-carotene and 7-dehydrocholesterol) that do not possess vitamin activity but which can be converted into active vitamins by the body. The ability of the body to store reserves of vitamins also varies. For example, while vitamin A stores in liver can last for 2–6 months and reserves of vitamin B12 can last for a year through enterohepatic cycling, reserves of thiamin may be sufficient for only 1–2 weeks (Saastamoinen & Harris 2008).
The vitamin contents in feeds and forage typically decrease during storage. As many horses do not have access to good quality, fresh green forage all year round, vitamin supplementation is often required to meet requirements. For this reason industrially produced vitamins are often added to horse diets in order to supplement the intake of natural vitamins. Although this practice is sound, it does create the potential for over supplementation and toxicity. In one survey of feeding practices in racing stables, vitamin A and D were often overdosed (up to more than 6 times above the requirement) while the vitamin E supply just met requirements (Meyer et al 1991). This means that when discussing appropriate vitamin provision one needs to cover not only the requirements on an individual basis but also the prevention of undesirable toxic effects, especially if multiple vitamin-containing supplements are fed.
General considerations regarding vitamin requirements
Derivation of requirement recommendations
Vitamin requirement figures are usually derived from factorial calculations or dose–response relationships. The predominantly functional nature of vitamins would seem to make the latter the method of choice. However, there are very few good quality, published data sets that can be used for deriving vitamin requirements of horses; response curves are often based on too few data and typically extrapolations from other species are made. In addition, several internal factors (e.g., animal age, amount of exercise and reproductive status) as well as external factors influence the amount of an individual vitamin needed for the particular metabolic or immunologic function in question, which complicates the characterization of a clear dose–response curve. External factors, such as the type and quality of the diet and the amount of access to sunlight, also need to be taken into account when considering an individual animal’s requirements. This can be achieved by increasing or reducing the more general requirements in response to the individual circumstances e.g. due to the known importance of vitamin B1 for carbohydrate metabolism (Bates 2001) it can be assumed that sport horses being exercised at a given level would need more vitamin B1 when on a high-starch diet than when consuming a high-fat feed. Vice versa, it is likely that horses on a high-fat diet would benefit from a higher intake of vitamin E. Various health problems such as recurrent airway obstruction (RAO), nephropathy or gastrointestinal diseases can also modify the utilization of, and therefore the need for, several vitamins. However, this is not an issue that should be considered when formulating requirement figures, rather the health status should be taken into account when adapting the respective requirement figures for the individual circumstances.
Another question is to decide whether vitamins that can be formed through metabolism (such as vitamin C) or produced by gut microbes (vitamin K and vitamins of the B-complex) also need to be given via the feed. The extent of the absorption of vitamins derived from microbial production is largely unknown. The potential for vitamin synthesis is large given the size of the hindgut fermentation vat. However, the extent to which vitamins are absorbed from the caecum and large intestine is not known. Nonetheless, it is evident that horses can survive without additional oral intake of vitamin K or vitamins of the B-complex. It may be that these vitamins are produced in sufficient quantities by microbes in the terminal small intestine and absorbed there or that there is sufficient vitamin absorption from the hindgut (Schenk & Kolb 1982). However, it cannot be excluded that the status of those vitamins can be low under some circumstances of modern horse husbandry in the absence of dietary supplementation. It may also be that changes in the ration, strenuous exercise or other stressful conditions cause alterations of the microflora resulting in inadequate production of B-vitamins (Linerode 1967).
Experimental data from horse studies do not enable robust derivation of vitamin requirements that take into consideration all the major modifying factors. However, by using multiple smaller data sets with different response variables (e.g., serum kinetics after vitamin ingestion and minimal vitamin intake required to prevent critical conditions such as night blindness), requirements have been estimated for vitamins A, D, E, thiamin and riboflavin (NRC 2007). It should be noted that the absolute requirement for a given vitamin may differ depending on the test variable used. For vitamin K, most vitamins of the B-complex and vitamin C, experimental data do not allow development of definitive recommendations for dietary intakes. An exception is made for thiamin and, using less rigorous demands for scientific verification, also for riboflavin. Vitamin K, biotin and vitamin C are discussed in this chapter with respect to the interest in these vitamins; however, precise dietary requirements are not given for these vitamins. Because of a lack of scientifically-derived data in horses, the other B-vitamins are discussed only very briefly in this chapter.
Rationale
Vitamin requirements have mostly been derived from studies that have used horses of a medium body size. The practical use of such requirement figures when expressed per unit of body weight (BW) may work quite well when the weight range of the horses from the original studies covers the BW of the target animal. The problem is, however, the remarkably high BW variability of equines: ranging from less than 100 kg in miniature Shetland ponies to 1000 kg and more in Shire horses. The same problem occurs with dogs (NRC 2006). Extrapolating BW-based requirements to larger-sized animals may lead to an unrealistic overestimate. Based on such extrapolations, for example, a Shire horse would need a much higher nutrient density (per unit of energy) in the feed than a medium-sized horse in order to cover maintenance requirements but this does not fit with practical experience.
Maintenance energy requirement is an alternative to consider as the basis for vitamin requirements, but again this is not a viable option due to wide variation in energy needs. Depending on breed, training status and body composition, energy needs may range from 0.40 to 0.64 MJ of metabolizable energy per kg of metabolic BW (BW0.75) with additional variance of ±10% associated with spontaneous activity and temperature regulation (Kienzle et al 2010). Taking a mean conversion rate of 83%, which may fit well for hay-based feeding rations supplemented with limited amounts of concentrates (Kienzle & Zeyner 2010), this would be equal to 0.48 to 0.77 MJ (~60% variation) of digestible energy per kg of BW0.75. There is no evidence that vitamin requirements for healthy animals at maintenance vary by this amount.
Using maintenance DM intake as the basis for vitamin requirements is also not promising because either the DM intake is assumed to be ~2% of the BW (GfE 1994, NRC 2007), which preserves the problems that occur when BW is taken as the basis, or the DM intake is taken to be that which is needed to provide the maintenance energy requirement for that individual. The latter also can lead to confusion, e.g. there is no evidence that the vitamin requirement is significantly modified when obese horses are being fed a restricted diet with low energy intake.
Rucker (2007), however, suggests that the amount of specific substances (which are generally thought to be nutritionally essential or conditionally important) needed per day is similar among animal species (or in animal species with a considerably high variation in BW) when expressed per BW0.75. Theoretical considerations on this subject were developed more than 60 years ago (Brody 1945, Lucky 1951). The rationale is that it is more likely for nutrients involved in biochemical reactions (e.g., as catalysts or cofactors) to be linked to basal metabolism (expressed as 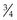 power of BW) rather than to absolute BW (Rucker & Storms 2002, Rucker & Steinberg 2002). For example, Grollmann & Lehninger (1957) described the potential synthesis of vitamin C from D-glucuronic acid, for which glucose and galactose serve as precursors. Vitamin C synthesis in vivo obviously cannot be greater than the amount of glucose and galactose shunted through the oxidative pathway which is essential for vitamin C synthesis (Grollmann & Lehninger 1957, Rucker et al 1980, Rucker & Steinberg 2002). When expressed relative to the BW0.75, extrapolation from the available animal data yields values that are basically in keeping with the human requirement (Rucker 2007). Accordingly, it is suggested that in addition to basal daily energy utilization, allometric scaling based on BW0.75 may be used to predict and compare basal nutrient requirements among widely varied species, as well as make comparative toxicological and other biological assessments and predictions (Rucker 2007).
power of BW) rather than to absolute BW (Rucker & Storms 2002, Rucker & Steinberg 2002). For example, Grollmann & Lehninger (1957) described the potential synthesis of vitamin C from D-glucuronic acid, for which glucose and galactose serve as precursors. Vitamin C synthesis in vivo obviously cannot be greater than the amount of glucose and galactose shunted through the oxidative pathway which is essential for vitamin C synthesis (Grollmann & Lehninger 1957, Rucker et al 1980, Rucker & Steinberg 2002). When expressed relative to the BW0.75, extrapolation from the available animal data yields values that are basically in keeping with the human requirement (Rucker 2007). Accordingly, it is suggested that in addition to basal daily energy utilization, allometric scaling based on BW0.75 may be used to predict and compare basal nutrient requirements among widely varied species, as well as make comparative toxicological and other biological assessments and predictions (Rucker 2007).
Key Points
• Vitamin requirements for both maintenance and production/performance are best expressed as a function of metabolic body weight (BM0.75).
• The NRC and many other reference books still use bodyweight as the basis for vitamin requirement recommendations; this approach results in unrealistic overestimates in larger-sized horses.
Life stage and life style
Maintenance vitamin requirements need to be adjusted for the additional needs associated with production, for example the transfer of vitamins to the fetus or into milk. Lack of data concerning the optimal quantity of vitamin in the target tissue and the transfer rate from the consumed vitamin to the respective product hampers use of the factorial method. For example, weighted means of 2077 and 128 international units (IU) of vitamin A (n = 159) and D3, (n = 87), respectively, per kg of mare milk have been extracted from the literature (Coenen et al 2010). Such data may serve as a basis for calculations, but it is not known whether these numbers represent ideal milk vitamin content or just values found in certain management systems. Furthermore, while orally provided vitamin A may increase serum retinol to a certain extent (Sklan & Donoughue 1982) even very high intakes of vitamin A do not automatically result in significant enrichment in the mares’ milk (Großer et al 1995).
Fat-soluble vitamins
Vitamin A and β-carotene
Vitamin A belongs to the group of retinoids. However, the various retinoids and carotinoids which are precursors of vitamin A differ considerably in their biological activity. One international unit (IU) of vitamin A is equivalent to the biological activity of 0.300 µg all-trans-retinol, 0.334 µg of all-trans-retinyl acetate, 0.359 µg all-trans-retinyl propionate or 0.550 µg of all-trans-retinyl palmitate for example (Seehawer & Schliffka 2006).
Major biological functions
Vitamin A plays an important direct role in vision (Wald 1968). It helps to regulate cell differentiation by influencing gene expression (Solomons 2001) and to maintain epithelial surfaces as well as the innate and the adaptive immune response (Kolb 1995, Stephensen 2001). The last two functions represent the key importance of vitamin A in the defense against infection. However, the most crucial role vitamin A plays is in the reproduction process. Both deficiency and oversupply result in impairment of fertility and may cause severe teratogenic effects in mammals (for review see Schweigert 1998a, b). Retinoic acid exerts its effect in a similar manner to a steroid hormone via nuclear receptors. Thus, vitamin A has a key role in various endocrine regulatory systems, which explains the numerous effects of this vitamin, including its role as a morphogen in embryonic development.
Whether β-carotene has a vitamin A-independent essential biological function is not known. However, in contrast to vitamin A, β-carotene serves as an unique antioxidant and a highly efficient single oxygen quencher (Bendich 1989).
Sources
Vitamin A
Vitamin A is available only from feed supplements that contain this vitamin in the form of retinyl-esters such as retinyl-palmitate or retinyl-acetate. They are thought to be hydrolyzed to retinol in the small intestine and absorbed there (Schweigert 1998b). According to one study (Bjondahl & Virkki 1977), the absorption of vitamin A is more rapid with xylitol and polyol as a vehicle than if water is used. After absorption, the majority of the vitamin will be transported to the liver and stored there or distributed via the blood stream to target tissues. In the blood, retinol occurs in a protein-bound form. The release of vitamin A from the liver is assumed to occur in response to demand by peripheral tissues (Schweigert 1998a). Jarrett and Schurg (1987) described an increase in plasma concentrations of vitamin A four hours after intake. However, despite the fact that plasma concentrations of retinol and retinyl esters respond to oral intake (Sklan & Donohue 1982, Jarret & Schurg 1987), plasma or serum levels do not adequately reflect the overall vitamin A status of the horse because hepatic vitamin A stores maintain blood retinol homeostasis in the face of changing dietary supply (Bondy & Sklan 1984, Jarrett & Schurg 1987, Mäenpää et al 1988a, Saastamoinen & Juusela 1992, Greiwe-Crandell et al 1997). Meyer et al (1995) did not detect a plasma response to oral vitamin A but did observe increased liver retinol levels. Measurement of the serum retinol response after a period of depletion may provide a more sensitive indication of overall vitamin status (Greiwe-Crandell et al 1995, 1997).
β-carotene
Natural feedstuffs for horses do not contain vitamin A but contain varying amounts of carotenoids that act as provitamins. The most important of these vitamin A precursors is β-carotene. Apart from yellow corn (where not all the color comes from β-carotene) most complementary feeds or concentrates only contain negligible quantities of β-carotene. However, this provitamin can be found in large amounts in green grass and in decreasing quantities in artificially dried grass followed by grass silages and then hay (Table 9-1).
Table 9-1 Guide to the Mean (min – max, in mg/kg of Air Dried Material) Levels of ß-Carotene and Vitamin E in Selected Types of Roughage (AWT 2001)
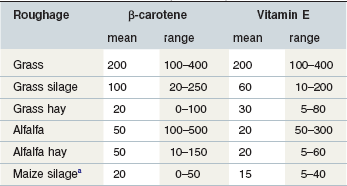
For horse rations, therefore, fresh grass and good-quality leafy hay are the most important natural sources of vitamin A (retinol) (Fonnesbeck & Symons 1967). Corn provides only about 1/8th the amount of β-carotene when compared to green forage. Factors affecting the content of β-carotene in pasture include the plant species, climatic conditions, and stage of maturity. Ballet et al (2000) described mean values of about 278 vs. 59 mg of β-carotene per kg of DM in vegetative vs. mature grasses. The leaf-to-stem ratio is important because leaves have much higher levels of β-carotene. Carrots provide high concentrations of β-carotene but there are differences between varieties. In general, β-carotene content is positively associated with intensity of color. However, crop fertilization practices further influence the carotene content (Evers 1989). Beta-carotene may be absorbed intact and then converted to retinol or can be converted to retinol or retinyl-esters in the intestinal mucosa (Ullrey 1972, Bondy & Sklan 1984, Napoli 2000).
Bioavailability
In humans, ~90% of retinol is absorbed but only ~3% of carotenoids (Sate & Patel 2010). Similar to the other fat-soluble vitamins, retinol requires bile acids for emulsification and micelle formation in order to be absorbed into enterocytes. Within the cell, retinol is re-esterified and packaged into chylomicrons which then enter the lymphatic system. Retinol is primarily removed by the liver where it is stored within the hepatic stellate cell (Sate & Patel 2010). It is presumed that a similar process occurs in the horse, although the role of chylomicrons remains uncertain due to the controversy over their presence in adult horses (see Chapter 7).
The bioavailability of synthetic β-carotene in horses is another topic that has not been satisfactorily examined. Greiwe-Crandell et al (1997) suggested that retinyl-palmitate (72 000 IU vitamin A), but not a vitamin A equivalent water-dispersible-carotene preparation, given in two large doses per week was effective for improving the vitamin A status (in terms of serum retinol and relative dose response) of mares throughout a 20 month repletion period following 8 months of depletion. A similar water-dispersible preparation of β-carotene given in daily doses of 1.8 mg β-carotene/kg BW also failed to alter plasma β-carotene concentration (Watson et al 1996, Eitzer & Rapp 1985). Kienzle et al (2002) reported an increase in plasma β-carotene concentrations when either natural β-carotene or a synthetic beadlet preparation was fed at half the amount (0.8 mg β-carotene/kg BW) given by Watson et al (1996). However, different synthetic preparations of both vitamin A and β-carotene, such as beadlets, coated and spray-dried products, may differ in terms of their dispersion within aqueous solutions. It is therefore likely that the absorption of synthetic β-carotene depends on the preparation used, which means that the effects of one preparation may not be directly inferred from the results of another. Plant oils may facilitate the transfer of fat-soluble vitamins and pro-vitamins to micelles and, thus, enhance absorption rate.
Effect of concurrent oil administration
According to expectations, Zeyner et al (1995) found a substantially higher concentration of β-carotene in the blood serum of horses fed a diet of meadow grass vs. grass hay. Interestingly, the corresponding differences in serum retinol levels appeared to be affected by supplemental soybean oil (Fig. 9.1). Kienzle et al (2002) described an increase in serum β-carotene when sunflower oil was added (4.7% of the diet) to the diet. Zeyner et al (1995) did not find an effect of 0.33, 0.66, 1.00, and 1.33 g of soybean oil per kg of BW/day on plasma retinol but did show that serum β-carotene concentrations were significantly elevated with the highest oil feeding level. In contrast, feeding a concentrate with 15% partly hardened soybean oil did not have an effect on the serum concentration of retinol and ß-carotene when given together with hay or with meadow grass (Fig. 9.1). However, in the study by Zeyner et al (1995) β-carotene came from natural sources only, whereas Kienzle et al (2002) provided supplemental β-carotene as a synthetic beadlet preparation.
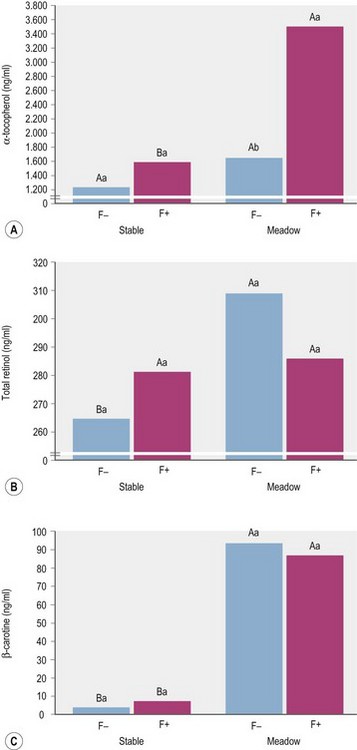
All horses were given 69 000 IU vitamin A and 1725 mg vitamin E per day by a mineral-vitamin-supplement. (ab different small letters indicate with p < 0.05 significant differences between means within “stable” or “meadow”; AB different large letters indicate with p < 0.05 significant differences between F− or F+; ± pooled SD for α-tocopherol, total retinol and β-carotene: 108.6, 33.9 and 4.71; Zeyner et al 1995.)
Conversion
Horses convert β-carotene to vitamin A rather inefficiently with an average conversion rate of about 33% (NRC 2007). Many factors such as age, activity, environmental conditions and vitamin A status may influence this conversion rate (Bondy & Sklan 1984). Studies in rats, for example, suggest higher conversion rates in pregnant than in growing animals (McDowell 1989). In mares, Schweigert & Gottwald (1999) found a marked increase in plasma levels of β-carotene (absolute values and in relation to the concurrently measured increase in vitamin A) between day 42 to day 2 antepartum. Possible reasons include an improved absorption of carotene and/or a reduced conversion into vitamin A, as well as a mobilization from tissue storages or a reduced uptake into tissues other than the mammary gland. However, the conversion rate from β-carotene to vitamin A seems to decrease when animals consume particularly large amounts of β-carotene (Ullrey 1972, Bondy & Sklan 1984, Solomons 2001). Although this has not been explicitly studied in horses it makes sense from a physiological point of view to avoid oversupply and possibly adverse effects. Furthermore, β-carotene utilization in horses seems to be affected by the type of forage fed, as indicated by more stable plasma vitamin A concentrations when alfalfa hay, which provides a lower intake of carotene, was fed (Fonnesbeck & Symons 1967).
Despite the fact that the suggested levels for conversion range between 300–555 IU of vitamin A per mg of β-carotene, it is generally assumed that 1 mg of the provitamin will be metabolized into 400 IU of vitamin A (NRC 1989, GfE 1994).
Stability
Vitamin A
The stability of vitamin A in the feed depends upon the formulation used and the methods of protection employed (Coelho 1996, McDowell 2000). Vitamin A can in principle be degraded through oxidation, prolonged storage, high temperatures during pelleting, catalytic effects of trace minerals as well as the peroxidizing effects of rancid fats (McDowell 2000). Although retinyl-acetate is susceptible to oxidation due to its five double bonds, synthetic sources of vitamin A are said to be more stable and thus less likely to be degraded during storage. Between 30 and 40% of the vitamin A present at mixing may be destroyed through the process of pelleting (McDowell 2000). Furthermore, the inclusion of trace minerals in premixes increases the risk of oxidation. The 2-month-stability of crosslinked vs. not crosslinked vitamin A within a prepared mixed feed was 90% vs. 65% (Seehawer & Schliffka 2006). Environmental conditions also are important. In one study, vitamin A was reduced to around 2% of the starting value when stored under high temperature and high humidity conditions over a period of 3 months compared to 88% under conditions of low temperature and low humidity (see McDowell 2000).
β-carotene
Carotenes appear to be one of the most difficult nutrients within forage to preserve (Fonnesbeck & Symons 1967; for review see Saastamoinen & Harris 2008). They may be destroyed by oxidation and this process is promoted by ultraviolet radiation and heat (Ballet et al 2000). In contrast to vitamin D, sun-cured hay has lower concentrations than fresh forage. It has been reported that the process of conserving forages can destroy around 80% of the β-carotene present and then biological oxidation continues at a rate of 6–7% per month during storage (McDowell 2000, Ballet et al 2000). Production of haylage leads to a loss of around 14% of the original β-carotene (Müller et al 2007) and further reductions occur during storage. Drying in the barn seems to preserve levels better, even though prolongation of the drying process may lead to similarly high losses (Ballet et al 2000). In addition, the loss of leaves also decreases the carotene content of hay. Certain legumes, particularly alfalfa and soybeans, contain the enzyme lipoxidase that unless quickly inactivated readily destroys much of the carotenes (McDowell 2000). Rapid dehydration can help preserve the carotene content of alfalfa (with 0–30% loss depending on residual moisture levels). In one study the β-carotene content of corn decreased to about 50% after 8 months of storage at 25oC and to 25% after 3 years (McDowell 2000).
Requirements
Maintenance
The vitamin A requirement for maintenance derives from the intake needed to prevent night blindness plus an extra need to secure tissue storage (NRC 1989). Guilbert et al (1940) suggested, based on a depletion-repletion study with growing Percheron horses, a minimum intake of 17 to 22 IU vitamin A/kg BW/day to be necessary to prevent night blindness. The same group reported that about three times this amount (51 to 66 IU/kg BW/day) was needed for appropriate tissue storage in rats. Taking daily vitamin A intakes of horses without night blindness (22.9 ± 5.1 IU/kg BW/day, n = 10) from Guilbert et al (1940) and adding two standard deviations comes close to the vitamin A requirement for maintenance given by NRC (1989): 30 IU/kg BW. This is similar to the 150 IU/kg BW0.75 recommended in Table 9-2.
Table 9-2 Requirements (GEH) for the Daily Vitamin Supply for Horses of Different Physiological States (per kg of BW0.75)
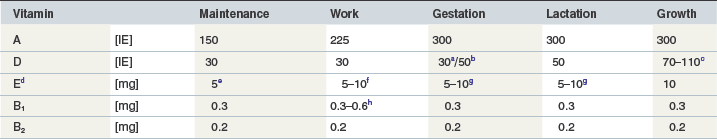
c In IU/kg BW0.75: 0–6 month: 110, 7–12 month: 90, 13–18 month: 80, 19–24 month: 70 and 25–36 month: 50.
d Expressed as DL-α-tocopherol-acetate (1 mg = 1 IU).
e When a high-fat diet is given, which is unlikely under maintenance conditions the horse should receive 10 mg/kg BW0.75/day.
f The higher quantity should be given when a high-fat diet is fed and in horses exercised more intensively. According to empirical experiences it is conceivable that horses susceptible to myopathy such as equine rhabdomyolysis syndrome (Harris 2005b) and particularly heavily exercised horse may profit from a significantly higher supply of around 20 mg/kg BW0.75 per day.
g Under certain conditions (high infection pressure or overall critical health status) it may be beneficial to provide 10 mg of vitamin E/kg BW0.75/day to mares in the last month of pregnancy and first month of lactation.
h Exercised horses with elevated carbohydrate metabolism should receive the higher quantity.
Exercise
Precise recommendations for vitamin A nutrition of the performance horse are not available. Gastrointestinal tract permeability and resistance to infection may be affected by exercise and this could be a rationale for increasing the recommendations for Vitamin A in athletic horses given its role in helping to support mucosal integrity and the immune system. It has been suggested, for example, that vitamin A deficiency impairs innate immunity by impeding normal regeneration of mucosal barriers damaged by infection and by diminishing the function of neutrophils, macrophages and natural killer cells (Stephensen 2001). The NRC (2007) stayed with the recommendation that the requirement for work lies somewhere between maintenance (30 IU/kg BW/day) and that for gestation and lactation (60 IU/kg BW/day): i.e., 45 IU/kg BW/day. Most recommendations in the literature vary between 45 and 90 IU/kg BW/day (NRC 2007, INRA 1990, GfE 1994). Transformed to metabolic body weight this corresponds to a daily vitamin A requirement for work of 225 IU/kg BW0.75 (Table 9-2).
Pregnancy and lactation
Several studies reported seasonal variations in vitamin A status in broodmares (Mäenpäa et al 1988a, b, Greiwe-Crandell et al 1997). These variations may in part be due to changes in the diet i.e. access to pasture vs. feeding preserved forages (Fonnesbeck & Symons 1967, Greiwe-Crandell et al 1997). However, it has also been shown that the seasonal decline in the vitamin A status is more pronounced in pregnant than non-pregnant mares (Mäenpäa et al 1987, 1988a) indicating that vitamin A metabolism is higher during pregnancy. Furthermore, Stowe (1982) suggested that vitamin A utilization increases around parturition due to elevated secretion of the vitamin into colostrum. However, the seasonal decline in vitamin A status occurs in broodmares without (<0.8 IU vitamin A/kg BW/day) and with supplementation of vitamin A in the form of retinyl-palmitate (125 IU vitamin A/kg BW/day; Greiwe-Crandell et al 1997). Stowe (1967) suggested that oral or parenteral doses of a combination of vitamin A and vitamin E will improve reproductive state (e.g., more serviced heats and live foals). However, it should be noted that although vitamin A status of unsupplemented mares without access to pasture decreases, no negative effects due to this decline have been reported. To date there is no evidence suggesting that the vitamin A requirements for pregnant and lactating mares are different from those previously recommended by NRC (1989, 2007): 60 IU/kg BW/day), which is equal to 300 IU/kg BW0.75 (Table 9-2).
Whether horses have a need for supplemental β-carotene independent from that of vitamin A is controversial (Enbergs & Klemt 1987, Schubert et al 1991) with most work in this area focused on the pregnant and lactating mare. Some reports indicated that supplemental β-carotene may benefit the fertility of broodmares (for review see Enbergs & Klemt 1987). However, the described endocrinological and clinical effects of supplemental beta-carotene could not be clearly distinguished from a possible impact of additional vitamin A. In an attempt to resolve this problem, Schubert et al (1991) fed two groups of 12 Shetland pony mares a semi-purified diet that was free of β-carotene. After one year of depletion, the mares received over a period of 4 years either 12 000 IU (year 1 and 2) and 15 000 IU (year 3 and 4) of vitamin A per day (control group) or 150 mg of β-carotene plus 10 000 IU of vitamin A per day (year 1–4, treatment group). There was a tendency for the conception rate to be higher in the carotene-free group whereas embryo loss and abortion tended to be lower in the carotene-supplemented mares. The concentrations of progesterone, pregnant mare serum gonadotropin, thyroxine, and triiodthyronine in the blood were unaffected by the dietary treatment. However, total protein and several protein fractions (α- and β-globulin) in the mares’ serum as well as the foals’ birth weight (in % of the weight of the dam) and the weight gain till the 5th month of life were significantly higher in the carotene-supplemented group. Whether these outcomes would have been achieved by just providing more vitamin A supplementation remains unclear. These results do not support the opinion that supplemental β-carotene impacts fertility although there may be benefits to foal health. Enbergs & Klemt (1987) found that the duration of neonatal foal diarrhea was inversely correlated with serum β-carotene concentration of the mares. In contrast, Kuhl et al (2011) reported that supplementation of Warmblood broodmares at 1000 mg of β-carotene per day from 14 days before the expected date of foaling to 42 days after parturition did not affect the incidence of foal diarrhea. Carotene intake via the core diet, however, was not reported in this study.
Further work is needed to clarify the effect of pre- and postpartum diet on the foal’s vitamin A status. One study showed that plasma β-carotene is highly correlated (r2 = 0.9) with concentrations in the mare’s colostrum (Schweigert & Gottwald 1999). Interestingly, levels of β-carotene in the colostrum were 65 times higher compared to milk collected later in lactation while concentrations of vitamins A and E were only 3 to 6 times higher. However, the addition of 20 000 or 100 000 IU vitamin A to a basal diet (hay, oats, and mineral supplement) of Haflinger mares did not change the vitamin A concentration of colostrum (Großer et al 1995). Zeyner et al (2004) demonstrated a substantial decrease in serum concentrations of total retinol (from first sucking until 6 h after birth) and β-carotene (until 12 h after birth) in trotter mares whereas serum retinol in the foals increased over time (Fig. 9.2). Although the mares’ β-carotene pool was apparently utilized, no corresponding increase in serum β-carotene of the foals was detected. In fact, serum β-carotene activity in the foals was below the limit of detection of the assay. One reason may be that mares’ β-carotene pool was in part used to maintain their own serum retinol concentrations (Fig. 9.2). Alternatively, the serum concentrations of β-carotene in this study were particularly low, compared to other published data from mares around parturition (Schweigert & Gottwald 1999). This in turn was probably due to dietary deficiencies resulting from feeding hay that had been stored for a long period together with a mixed feed that was not fortified with β-carotene or vitamin A. This supports the need to provide pregnant mares with an adequate vitamin A intake throughout gestation – supplementation will become particularly important if the dam is kept inside and fed very mature forage during the final stages of gestation.
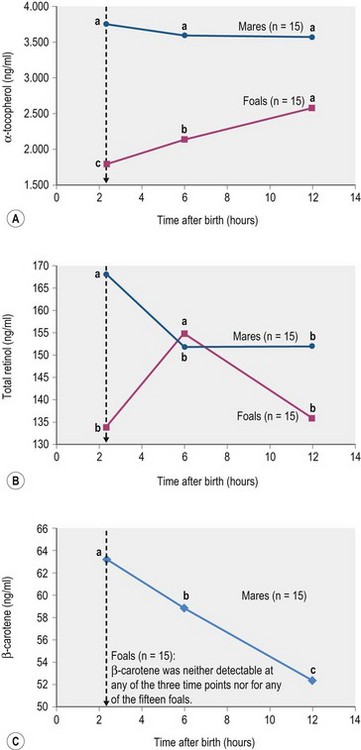
(abc different small letters indicate with p < 0.05 significant differences between means within mares or foals; ± pooled SD for α-tocopherol, total retinol and β-carotene: 203.0, 14.8, and 5.37 [mares only]; Zeyner et al 2004.)
Growth
Guilbert et al (1940) reported no apparent problems with growth in Percheron horses (119 to 444 days old) consuming 22.9 ± 5.1 IU vitamin A/kg BW/day. Donoghue et al (1981), however, suggested from a study of 4- to 12-month-old ponies that a dose ranging from 60 to 200 IU/kg BW/day was necessary for optimal growth. Despite using younger horses (orphan foals), Stowe (1968) reported a minimum requirement of vitamin A that was substantially lower (9.5–11 IU/kg BW/day). For reasons of “security”, however, the daily intake of vitamin A recommended by the NRC 2007 for growing horses lies at the lower limit of the range given by Donoghue et al (1981); i.e., 60 IU/kg BW. On a metabolic body weight basis the recommendation is 300 IU/kg BW0.75 (Table 9-2).
Effects of deficiency and excess
Deficiency
In humans, vitamin A deficiency is most commonly seen with malnutrition or fat malabsorptive states (e.g., chronic liver disease; Sathe & Patel 2010). Based on data from several mammalian species, vitamin A deficiency may cause impaired growth resulting in developmental orthopedic disease (DOD), reduced protective function of epithelial cells, decreased efficiency of the immune system, disturbed maturation of spermatozoa, reduced storage of rhodopsin (which may cause night blindness), lowered secretion of glucocorticoids, and increased early embryonic mortality (Kolb 1995). The classical clinical sign of vitamin A deficiency is night blindness, which appeared when growing Percheron horses consumed 5 to 10 µg total carotene/kg BW/day (no more than 2 to 4 IU vitamin A/kg BW/day) for 265 to 627 days (Guilbert et al 1940). However, mares did not show any clinical signs even when they consumed hay with extremely low β-carotene (4 mg /kg of dry matter, DM) for 22 months (Greiwe-Crandell et al 1997). In orphan foals, signs of vitamin A deficiency were observed when feeding a semi-purified diet devoid of vitamin A activity (Stowe 1968). Impairments to growth and hematopoiesis may be the most sensitive indicators of deficiency (Donoghue et al 1981). Vitamin A is stored in the liver (McDowell 2000) and this reserve store may help to buffer a short-term deficiency in dietary intake. This means that serum retinol activity will not accurately indicate marginal vitamin A status. In contrast, the relative dose response (RDR) test is an indirect measure for liver vitamin A stores, potentially allowing a more precise description of vitamin A status (Greiwe-Crandell et al 1995). Overall, vitamin A deficiency is very rare in horses and only likely to occur under extreme conditions of deprivation (Frape 2000, NRC 2007).
Excess
Vitamin A toxicity may result in elevated bone fragility, hyperostosis, exfoliated epithelium, and teratogenesis (NRC 1987) as well as DOD in growing horses (Donoghue et al 1981, Kronfeld et al 1990). Doses of 160 IU/kg (Jarrett & Schurg, 1987) and 220 IU/kg BW (Saastamoinen & Juusela, 1992) have been fed for 9 months or more without harmful effects but also without beneficial effects. According to NRC (2007), the safe upper safe limit is 16 000 IU/kg dry matter (~360 IU/kg BW/day) although it was noted that this is most likely erroneous based on the absence of toxicity in horses consuming such amounts. Even though the intake of β-carotene from pasture could theoretically reach critically high levels if all the β-carotene was fully converted into vitamin A (with a conversion rate assumed to be 400 IU of vitamin A per 1 mg of β-carotene), toxic effects of β-carotene have not yet been reported in horses. This is thought to be due to a reduction in the conversion rate at high intakes.
Vitamin D
Vitamin D (calciferols) has two forms, vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol). Despite not being chemically identical, both are derived from steroids. They are formed from their respective pro-vitamins following exposure to ultraviolet radiation (UV-B radiation wavelength 290–318 nm). Ergosterol, found in plant material, is the precursor of vitamin D2. In the majority of domestic species, 7-dehydrocholesterol within the skin (which is synthesized through the animal’s metabolism) serves as the precursor to vitamin D3. In general, vitamin D3 has higher potency than vitamin D2 (Harrington & Page 1983) but there is species variation (Schenk & Kolb 1982). In humans, vitamin D from the skin (D3) binds to D-binding protein in the blood and is transported to the liver. Following emulsification and micelle formation ~80% of ingested vitamin D2 is absorbed by enterocytes in the duodenum and ileum, incorporated into chylomicrons and transported to the liver via the lymphatic system (Sathe & Patel 2010). To become biologically active, vitamin D must be activated via reactions in the liver (hydroxylated on carbon 25 forming calcidiol; 25-OH-D) and then in the kidney (synthesis of calcitriol via hydroxylated on carbon 1; 1,25-OH-D). The active form, 1,25-OH-D, should actually be classified as a hormone. In humans, vitamin D status appears to be best assessed by measuring calcidiol levels because with vitamin D deficiency, parathyroid hormone increases the renal production of calcitriol resulting in apparently normal concentrations (Sathe & Patel 2010).
In the majority of mammalian species, vitamin D plays a key role in regulation of calcium and phosphorus homeostasis, especially in bone. However, the importance of vitamin D is questionable in equines, not least because of the significantly low plasma levels of vitamin D activating products (Smith & Wright 1984, Mäenpää et al 1987, 1988b, Harmeyer et al 1992) and the absence of clear evidence of biological effects.
Major biological functions
In the majority of domestic species, vitamin D is suggested to be largely responsible for (1) stimulating the intestinal absorption of calcium, (2) the renal reabsorption of calcium and phosphorus (in the presence of the parathyroid hormone), (3) building up the bone matrix, and (4) the calcification of osteoblasts. The anabolic effect of vitamin D metabolites within bone results from a combination of (1) indirect suppression of bone resorption, (2) direct stimulation of osteoblastic matrix synthesis, and (3) osteoblastic bone formation (Bante et al 1996). This biological effect of vitamin D is linked to the presence of receptors, similar to those for steroid hormones, in different cells and tissues. This explains how vitamin D influences many other biological processes, including the growth and differentiation of epidermal cells, differentiation of cells of the hematopoietic system, immune modulation, in addition to the already mentioned influence on calcinogenesis (Kolb & Grün 1996).
Although Hintz et al (1973) reported that supplemental vitamin D promotes calcium and phosphorus resorption in horses, the actual impact of the vitamin on calcium metabolism in the horse seems to be rather low compared to other domestic animals (Harmeyer et al 1992). However, a particularly high protein level of a vitamin D-dependent receptor has been identified ex vivo in the duodenal wall of horses (Sprekeler et al 2011). This receptor is commonly known to be involved in an active, vitamin D-dependent transcellular pathway of Ca2+. The mRNA level of this receptor did not change remarkably throughout the intestine. Using chamber studies revealed Ca2+ active absorption in the duodenum but not in the cecum and specific sites of the colon (Sprekeler et al 2011). Horses therefore appear to have the capacity for active Ca2+ transport but passive mechanisms may be dominant. Breidenbach et al (1998) concluded that vitamin D does not seem to play a key role in regulating the homeostasis of calcium and inorganic phosphorus in horses under normal circumstances. However, the situation is different when pharmacological doses of vitamin D3 are given (Harmeyer & Schlumbohm 2004). Overall, it does appear that the metabolism and biologic functions of vitamin D in the horse differ in many respects from those of other mammalian species such as human beings, pigs or rats (Breidenbach et al 1998). For example, plasma levels of calcidiol (<10 nmol/l) and calcitriol (20–40 pmol/l) are very low even in healthy animals and would clearly be regarded as deficient in humans or pigs (Kaune & Harmeyer 1987, Harmeyer 1999, Sathe & Patel 2010). Furthermore, in vitro results indicate that, contrary to other species, equine kidney tissue does not synthesize the active form of vitamin D hormone from its precursor calcidiol (Harmeyer 1999). This area would benefit from further research.
Sources and bioavailability
How much vitamin D is formed in the skin (Webb & Holick 1988) depends on the duration and intensity of the sunlight and thus also on the geographical location as well as the season of the year (Nutrition Reviews 1989) and the type of housing management. Ultraviolet radiation tends to be low in the morning and during the winter season, due to the shorter daylight time. This is particularly true at latitudes above 50o which may be a problem when horses are trained in the morning and then housed for the remainder of the day (Saastamoinen & Harris 2008). It has been suggested that horses in such regions, especially in the winter season, may have a deficiency of vitamin D when not supplemented (Mäenpää et al 1987) as exemplified by seasonal changes in serum 25-OH-D. However, Saastamoinen & Juusela (1992) did not find such a seasonal variation nor an impact of dietary supplementation on serum levels.
In naturally preserved forages, some vitamin D2 will be synthesized from ergosterol if the forage is exposed, after cutting, to sunlight during desiccation (McDonald et al 1988, McDowell 2000). Because of reduced exposure to ultraviolet radiation, artificially dried hay will contain much less vitamin D2 than sun dried material (Ballet et al 2000: around 971 IU/kg DM when sun dried vs. 470 IU/kg DM when barn dried). As the highest quantity of vitamin D2 is found in dried leaves, the leaf to stem ratio is another influencing factor. The range of vitamin D2 content in selected forages from a study by Ballet et al (2000) is given in Table 9-3. As ergosterol is also present in other plant forms like fungi and yeasts, irradiated yeast can be used as a source of vitamin D2.
Table 9-3 Guide to Typical Concentrations of Vitamin D2 in Selected Forages (Ballet et al 2000)
| Roughages | Vitamin D2 (range, in IU/kg of DM) |
|---|---|
| Fresh green forages | 31–1800 |
| Dehydrated lucerne | 176–617 |
| Cattle silages | 80–866 |
| Hay | 90–5560 |
Both forms (D2 and D3) are used in the formulation of commercial complementary feeds and mineral-vitamin premixes, although use of vitamin D3 is most common. It has been suggested that vitamin D2 and D3 are absorbed at a similar rate from the gut, ~60–80% (Schenk & Kolb 1982). There is, however, a lack of studies explicitly addressing absorption rates in horses.
Stability
Inexpert mechanical handling of sun cured forages, especially hay, can lead to loss of leaf and thus of vitamin D. Furthermore, many other factors influence the stability of vitamin D in the feed including the presence of heavy metals, alkaline components, and exposure to light when O2 is present (Schenk & Kolb 1982). McDowell (2000) described a loss of 10 to 30% when complementary mixed feeds, which included mineral-vitamin premixes, were stored for either 4 or 6 months at 22°C.
Requirements
Based on blood levels, vitamin D status is low compared to other animal species (Smith & Wright 1984, Mäenpää et al 1987, 1988b, Harmeyer et al 1992). Supplemental vitamin D has been reported to increase calcium and phosphorus absorption in horses (Hintz et al 1973). However, no study has clearly shown the need for additional vitamin D on top of that synthesized in the skin through exposure to sunlight when horses are maintained under typical field conditions, including some pasture turnout. In situations when horses need to be kept inside (because of illness for example) or when the exposure to sunlight is restricted for other reasons, there may be a rationale for supplementation with vitamin D. Again without scientific evidence, it also is proposed that other life stage/life styles may benefit from the added security of knowing the animal has been provided with a known level of vitamin D through the diet. This may also be true for pregnant and lactating mares, when horses grow rapidly, or when young-stock come into training at an early age, all of which may increase vitamin D requirements. To ensure an adequate intake, vitamin D requirements for horses of different age and performance are given (Table 9-1) which are close to the recommendations published by GfE (1994) and NRC (2007). The main difference between these recommendations is the reference to BW0.75 instead of BW: 30 IE/kg BW0.75 for maintenance and early gestation (until the 7th month), 50 IU/kg BW0.75
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


