Chapter 11 Uvea
The uvea plays an important role in ocular physiology, and disorders of this tissue are common in veterinary practice. The iris controls the amount of light entering the eye, and the ciliary body alters the focal power of the lens, produces aqueous humor that supplies nutrition to ocular structures, and aids in regulating intraocular pressure (IOP). Together they also form a blood-aqueous barrier so as to maintain the clarity of the aqueous humor and vitreous. The choroid plays a major role in providing nutrition to the retina. Because of these diverse roles, uveal disorders are frequently associated with alterations in vision and IOP.
ANATOMY AND PHYSIOLOGY
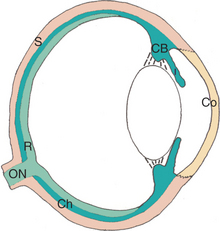
The eye consists of the following basic layers (Figure 11-1):
Iris
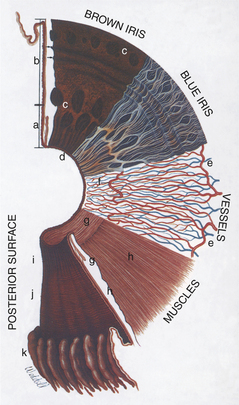
Viewed from the anterior surface, the iris has two zones, the pupillary zone (Figures 11-3 and 11-4) and the ciliary zone. A variable thickening of the iris at the junction of these two zones is called the collarette. The anterior surface of the iris is covered by a modified layer of stromal cells, the anterior border layer (Figure 11-5). The remaining parts of the iris are the stroma and sphincter muscle, the anterior epithelium and dilator muscle, and the posterior pigmented epithelium and pigment ruff. The posterior pigmented epithelium is continuous with the nonpigmented epithelium covering the ciliary body and eventually with the retina.
The bulk of the iris is stroma, which consists of fibrous connective tissue with bundles of collagen, pigmented and nonpigmented cells, and blood vessels in a mucopolysaccharide matrix. Variations in iris color are due to variations in pigmentation of the stroma and posterior pigmented epithelium and in the arrangement of the anterior border layer (Figure 11-6).
The temporal and nasal long ciliary arteries enter the iris near its root (see Figure 11-3) and form the major arterial circle, which may be incomplete. The vascular supply of the iris of domestic animals greatly exceeds that of the human iris. Therefore surgical procedures near the iris root in animals often result in profuse hemorrhage if the major arterial circle is transected.
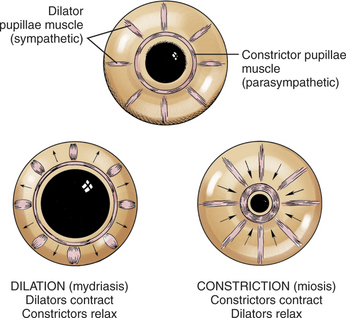
The dilator pupillae muscle extends as a continuous sheet in front of the anterior epithelium (see Figure 11-4) and is intimately related with it. The constrictor pupillae muscle is a flat ring of smooth muscle surrounding the pupil in the posterior iris stroma (see Figure 11-5).
Ciliary Body
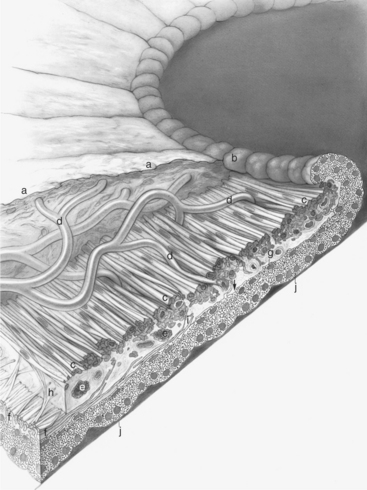
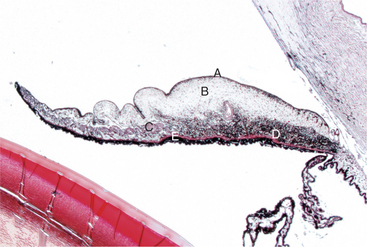
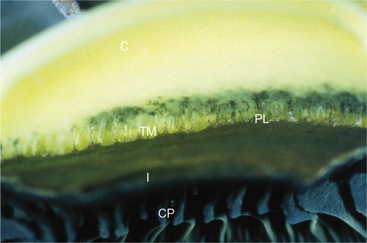
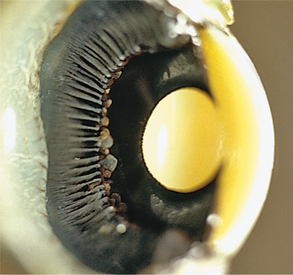
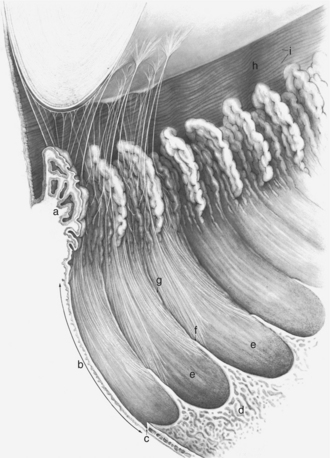
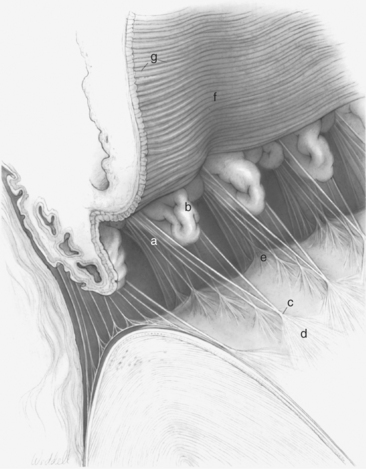
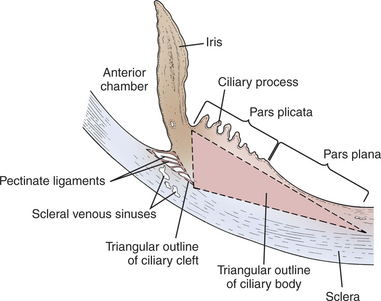
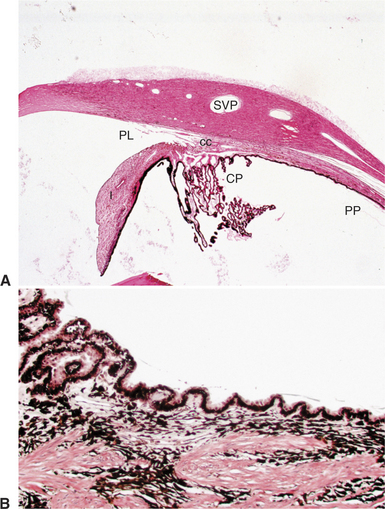
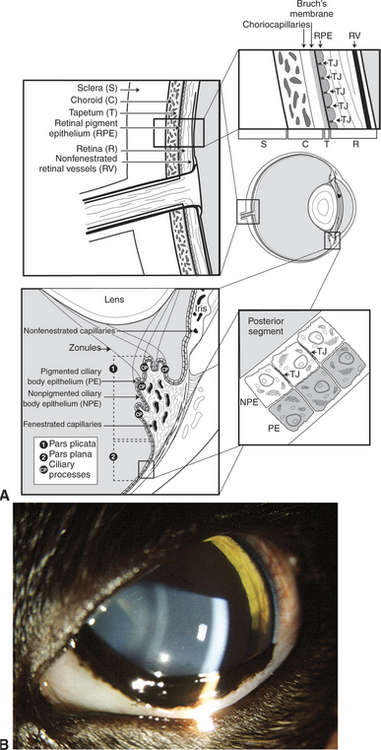
The ciliary body lies immediately posterior to the iris. On its posterior surface the ciliary body has numerous folds known as the ciliary processes (Figures 11-7 and 11-8). This area of the ciliary body, termed the pars plicata (folded part), merges posteriorly into a flat area (pars plana), which joins the retina. The zonular fibers, which support the lens, originate from the pars plana and between the ciliary processes (Figures 11-9 and 11-10).
Viewed in section, the ciliary body is triangular, with one side joining the sclera, one side facing the vitreous body, and the base giving rise to the iris and iridocorneal angle (Figure 11-11). The ciliary body is covered with two layers of epithelium, the inner layer of which is nonpigmented and the outer layer of which is pigmented. It is continuous with similar epithelium on the posterior surface of the iris and the pigment epithelium of the retina (Figure 11-12). The smooth muscle fibers of the ciliary muscle (parasympathetic innervation) together with blood vessels, connective tissue, and nerves occupy a large portion of the ciliary body (Figure 11-13). The muscle fibers originate near the apex of the triangle and insert into the region of the ciliary cleft and trabecular spaces of the iridocorneal angle. Contraction of the ciliary muscle causes the following:
Choroid
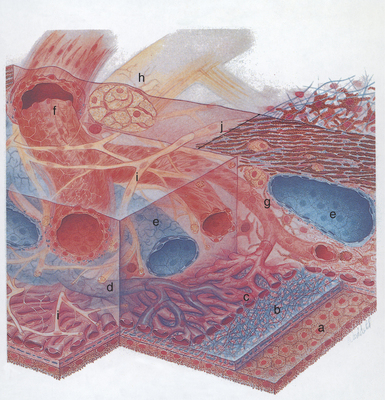
The choroid is a thin, variably pigmented, vascular tissue forming the posterior uvea. It joins the ciliary body anteriorly and lies between the retina and sclera posteriorly. The choroid is extremely vascular, with its capillaries arranged in a single layer on the inner surface to nourish the outer retinal layers (Figure 11-14). In species with limited retinal vasculature (e.g., horse, rabbit, guinea pig) the retina depends to a large extent on the choroidal blood supply. The choroidal stroma typically contains numerous melanocytes, which form a dark optical background to the retina. In most domestic mammals except the pig, a reflective layer—the tapetum lucidum—lies within the inner capillary layer. In large animals the tapetum is penetrated by numerous small capillaries, which appear as small focal dark spots (the stars of Winslow) when viewed end-on with the ophthalmoscope. The arteries and nerves to the anterior parts of the eye pass forward through the choroid. The choroid receives its main arterial supply from the following vessels:
Histologically the choroid consists of the following layers (see Figure 11-14):
In herbivores the tapetum is fibrous in nature (tapetum fibrosum), whereas in carnivores the tapetum is cellular and composed of reflective crystals (tapetum cellulosum) (Figure 11-15). The reflective properties of the tapetum, and not the presence of pigments, causes the distinctive color of the fundi of different animals and is the reason an animal’s eyes “shine” in the dark. This color varies with thickness of the tapetum, breed, age, and species. Reflecting light through the retina a second time improves the animal’s ability to function in dim light.
Blood-Ocular Barrier
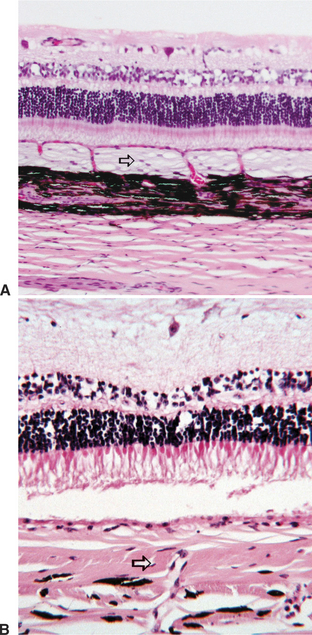
The uveal tract plays a key role in maintaining the blood-ocular barrier (Figure 11-16). Diseases involving the uveal tract frequently cause a breakdown of this barrier, which leads to exudation of excessive amounts of proteins or cells into the aqueous humor, vitreous, or subretinal space. The blood-ocular barrier is composed primarily of a blood-retinal barrier and a blood-aqueous barrier. The blood-retinal barrier is formed at the level of the retinal capillary vascular endothelium, which is nonfenestrated and has tight junctions, and the retinal pigment epithelium, which also has tight junctions and separates the relatively leaky choroidal blood vessels from the overlying retina. The blood-aqueous barrier is formed by tight junctions at the level of the nonfenestrated iridal vascular endothelium and between cells constituting the nonpigmented ciliary body epithelium. Most large molecules, especially proteins, are unable to pass through or between the cells in this barrier system. The exact anatomic location of the barrier is probably different for different substances (e.g., capillary endothelial cells, endothelial basement membrane, and intercellular junctions). By limiting the amount of protein and other large molecules that may scatter light in the aqueous and vitreous humor, these barriers serve to create a more optically perfect media. They are, however, frequently disrupted by inflammation or other disease processes.
PATHOLOGIC REACTIONS
Immune Mechanisms
CONGENITAL UVEAL ABNORMALITIES
Persistent Pupillary Membrane
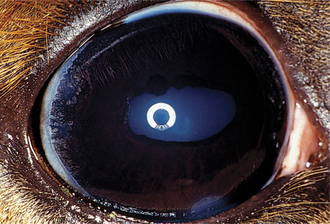
During development the pupillary membrane (anterior portion of the tunica vasculosa lentis) spans the pupil from one portion of the iris collarette to another and supplies nutrients to the developing lens (see Chapter 2). In dogs this membrane is usually resorbed during later fetal development and the first 6 weeks of life, leaving a clear pupillary aperture. It is not uncommon, however, for remnants to remain for several months or longer. In general small remnants spanning from one portion of the iris to another (iris-to-iris persistent pupillary membranes [PPMs]) have no visual consequences, although visual impairment may occur if strands contact the cornea (iris-to-cornea PPMs) or lens (iris-to-lens PPMs) and create an opacity within the visual axis (Figure 11-17).
PPMs occur in a large number of dog breeds, most notably the basenji, in which they are recessively inherited (see Appendix I). A genetic basis is also likely in many other dog breeds, but the mode of inheritance is probably not simply mendelian. PPMs may span from one region of the iris to another (sometimes crossing the pupil) or they may extend to the cornea or lens, creating opacities in these structures. PPMs can usually be differentiated from inflammatory anterior or posterior synechia on the basis of their origin near the iris collarette region (versus an origin at the pupillary margin for synechia) and their presence at birth. It usually is possible to see the membrane extending from the iris collarette region to the cornea or lens, although occasionally the membrane may have broken free and the cornea or lens opacity (often pigmented) is all that remains. Therapy is not typically required or possible. The best method of preventing the disorder is to examine breeding stock and breed only animals that are free of PPMs. Slit-lamp biomicroscopy is essential for the examinations.
Coloboma
A coloboma is a defect in the eye resulting from incomplete closure of the embryonic fissure. Typical colobomas occur in the inferomedial portion of the iris or choroid or adjacent to the optic disc (Figure 11-18). Colobomas of the sclera also occur in the collie eye anomaly. Although the embryonic fissure is not involved, coloboma is also applied to lid defects and to sector defects in the iris and lens.
Anterior Segment Dysgenesis
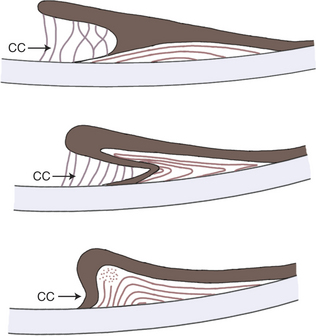
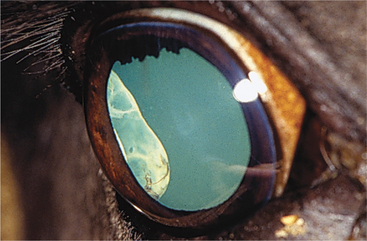
Anterior segment dysgenesis syndrome occurs frequently in Rocky Mountain horses and has two distinct ocular phenotypes: (1) large cysts originating from the temporal ciliary body or peripheral retina (Figure 11-19) and (2) multiple anterior segment anomalies, including ciliary cysts, iris hypoplasia, iridocorneal adhesions and opacification, nuclear cataract, and megalocornea (Figure 11-20). This condition may be codominantly inherited, so that ciliary cysts are seen in heterozygous animals and multiple anterior segment anomalies are seen in homozygous animals.
Disorders of Pigmentation
Heterochromia
Heterochromia refers to variations in iris coloration. Both eyes, one eye only, or only part of an iris may be affected, and often there are concurrent variations in coat color (Table 11-1). Heterochromia iridis refers to variations in pigmentation of different regions of the iris in the same eye (Figure 11-21), and heterochromia iridium refers to variations in coloration between the two eyes of the same animal. Although heterochromia may be normal, blue iridal tissue has also been associated with iris hypoplasia, iris coloboma, and corectopia as well as with absence of or a small tapetum and lack of pigmentation of the nontapetal fundus. An association between congenital deafness and heterochromia has also been recognized in blue-eyed white cats and in the Dalmatian, Australian cattle dog, English setter, Australian shepherd, Boston terrier, Old English sheepdog, and English bulldog.
Table 11-1 Breeds Affected by Heterochromia Iridis
| SPECIES | BREED | CHARACTERISTICS |
|---|---|---|
| Cat | Siamese | Subalbinism |
| Burmese | Variable iris hypopigmentation | |
| Abyssinian | Variable iris hypopigmentation | |
| Persian | Variable iris hypopigmentation | |
| Dog | Australian cattle dog | Dappling |
| Australian shepherd | Merling | |
| Boxer | White coat | |
| Collie | Merling (autosomal dominant) | |
| Great Dane | Harlequin coat (autosomal dominant) | |
| Long-haired dachshund | Harlequin coat (autosomal dominant) | |
| Dalmatian | Dappling (autosomal dominant) | |
| Malamute | Dappling | |
| Old English sheepdog | Heterochromia iridis | |
| Siberian husky | Dappling (autosomal dominant) | |
| Weimaraner | Iris hypopigmentation varies | |
| Horse | Pinto, appaloosa, white and gray horses | Variable heterochromia |
| Cattle | Hereford, shorthorn | Albinism, subalbinism |
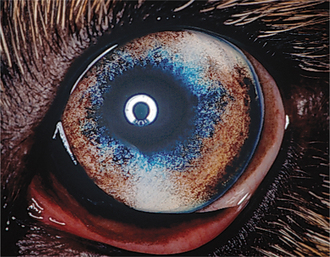
Figure 11-21 Heterochromia iridis (blue and brown iris) in an otherwise normal Australian shepherd dog.
Lay terms for heterochromia are as follows:
In most species heterochromia is of no clinical significance. In cattle ocular albinism has been further subdivided (Table 11-2).
Table 11-2 Ocular Albinism in Cattle
| TYPE OF ALBINISM | FEATURES |
|---|---|
| Partial | Iris blue and white centrally, brown peripherally |
| Hair color normal | |
| Incomplete | Iris light blue, gray, and white |
| Hair color white | |
| Some brown sectors in iris, and some colored hair patches | |
| Nontapetal fundus incompletely pigmented and choroidal vasculature visible | |
| Complete | Iris very pale blue or white |
| Hair pure white | |
| Variable fundus colobomas and tapetal hypoplasia |
Iris Nevus
Iris nevi (Figure 11-22) are most commonly observed in cats and dogs. They may consist of focal spots of hyperpigmentation. They must be differentiated from neoplasms that require surgical treatment. Iris nevi do not protrude above the surface of the iris and do not enlarge. Nevi have a low malignant potential and show an increase in the number of cells or greater pigmentation of existing cells. They must be observed carefully for changes, especially in cats, in which they may transform into the early stages of diffuse malignant iris melanoma.
UVEITIS
Clinical Signs
Clinical signs more specific for uveitis are as follows:
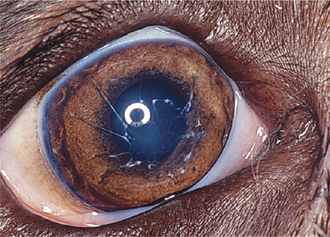
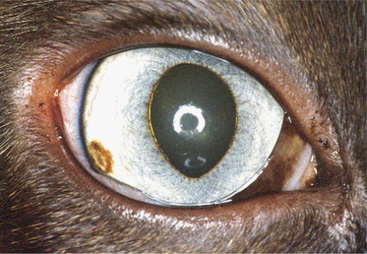
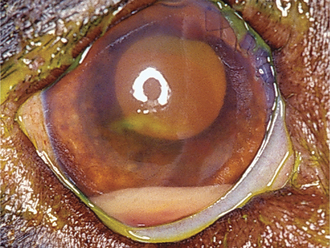
Aqueous flare is due to breakdown of the blood-aqueous barrier with increased permeability of vessels in the iris and ciliary body, resulting in release of protein into the aqueous. Keratic precipitates (KPs) are accumulations of inflammatory cells (neutrophils, lymphocytes, or macrophages) that adhere to the corneal endothelium. In large numbers these cells form a white layer in the anterior chamber called hypopyon (Figure 11-23). KPs may be small and scattered (in feline infectious peritonitis) or large and yellow (“mutton-fat” KPs) in granulomatous diseases. Miosis may be due to iridal edema or spasm of the iridal sphincter muscle. As the inflammation subsides, synechiae may form, causing an irregularly shaped pupil (Figure 11-24) or a scalloped appearance on dilation, with pigment remnants on the anterior lens capsule. If posterior uveitis is present, the vitreous may become hazy, and retinal edema, exudates, or detachments may be seen.
Sequelae of Uveitis
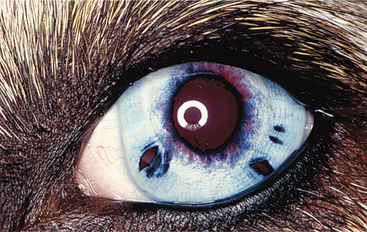
Posterior Synechiae
Posterior synechiae occur when fibrinous adhesions form between the lens and iris, with fibrovascular organization occurring later (see Figure 11-24). Formation of synechiae is more likely when aqueous protein content is high. If synechiae form around the entire circumference of the pupil, iris bombé occurs, preventing aqueous flow to the anterior chamber, and secondary glaucoma almost invariably follows. An irregularly shaped pupil is frequently caused by synechiae. If blood or exudate organizes in the anterior chamber, a connective tissue membrane may occlude or obliterate the pupil.
Retinal Detachment
Exudation and cellular infiltration from the choroid may cause retinal detachment.
Diagnosis of Uveitis
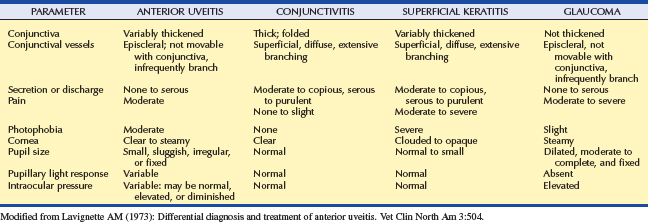
Anterior uveitis is distinguished from conjunctivitis, superficial keratitis, and glaucoma, the other causes of the red-eye syndrome (Table 11-3). The uvea is involved in numerous systemic disorders (Table 11-4). Such diseases usually affect other parts of the eye in addition to the uvea and are discussed in Chapter 18. Once uveitis is detected, every effort should be made to identify a specific cause of the inflammation so that the most effective therapy may be started. A thorough history and complete physical examination are essential for the proper diagnosis as to the cause of the inflammation in a given patient.
| CAUSE | MOST COMMONLY AFFECTED SPECIES |
|---|---|
| NEOPLASTIC/PARANEOPLASTIC | |
| Lymphosarcoma | Any |
| Melanoma | Dog, cat |
| Histiocytic proliferative disease | Dog |
| Hyperviscosity syndrome | Dog |
| Granulomatous meningoencephalitis | Dog |
| Miscellaneous primary intraocular tumors | Any |
| Miscellaneous metastatic tumors | Any |
| METABOLIC | |
| Diabetes mellitus (lens-induced uveitis) | Dog |
| Systemic hypertension | Cat, dog |
| Hyperlipidemia | Dog |
| Coagulopathies | Any |
| IDIOPATHIC | Any |
| IMMUNE-MEDIATED | |
| Cataracts (lens-induced uveitis) | Any |
| Lens trauma (phacoclastic uveitis) | Any |
| Immune-mediated thrombocytopenia | Any |
| Immune-mediated vasculitis | Any |
| Uveodermatologic syndrome (Vogt-Koyanagi-Harada–like syndrome) | Dog |
| INFECTIOUS | |
| Algae | |
| Geotricha spp. | Dog |
| Prototheca spp. | Dog |
| Bacteria | |
| Septicemia/endotoxemia due to any cause | Any |
| Leptospira spp. | Dog, horse |
| Bartonella spp. | Dog, cat |
| Borrelia burgdorferi | Dog, horse |
| Brucella spp. | Dog, horse |
| Escherichia coli | Cattle, horse |
| Streptococcus spp. | Horse |
| Rhodococcus equi | Horse |
| Listeria monocytogenes | Sheep, cattle |
| Haemophilus spp. | Cattle |
| Tuberculosis | Cattle, cat |
| Protozoa | |
| Toxoplasma gondii* | Any |
| Leishmania donovani | Dog |
| Ehrlichia canis or Ehrlichia platys | Dog |
| Rickettsia rickettsii | Dog |
| Yeasts and Fungi | |
| Aspergillus spp. | Chickens, turkeys, cat |
| Blastomyces spp. | Dog, cat |
| Coccidioides immitis | Dog |
| Cryptococcus spp. | Dog, cat |
| Histoplasma capsulatum | Dog, cat |
| Pseudallescheria boydii | Dog |
| Viruses | |
| Canine adenovirus types 1 and 2 (immune-mediated) | Dog |
| Canine distemper virus | Dog |
| Coronavirus (feline infectious peritonitis) | Cat |
| Feline leukemia virus | Cat |
| Feline immunodeficiency virus | Cat |
| Herpesvirus (Marek’s disease) | Chickens, turkeys |
| Herpesvirus | |
| Feline herpesvirus 1 | Cat |
| Canine herpesvirus 1 | Dog |
| Equine herpesvirus 1 and 2 | Horse |
| Ovine herpes virus 2 (MCF) | Cattle |
| Alcelaphine herpes virus 1 (MCF) | Cattle |
| Rabies virus | Dog |
| Equine influenza | Horse |
| Equine viral arteritis | Horse |
| Parainfluenza type 3 | Horse |
| MCF | Cattle |
| Parasitic | |
| Taenia multiceps | Sheep, dog |
| Echinococcus granulosis | Horse (rare) |
| Angiostrongylus vasorum | Dog |
| Dirofilaria immitis | Dog |
| Setaria spp. | Horse |
| Onchocerca cervicalis | Horse (equine recurrent uveitis) |
| Strongylus | Horse |
| Diptera spp. (ophthalmomyiasis interna) | Various |
| Toxocara spp., Baylisascaris spp. (ocular larval migrans) | Dog, cat Sheep/Goats, cat |
| Trypanosoma sp. | |
| Elaeophora schneideri | Sheep/Goats |
| TOXIC | |
| Drugs | |
| Pilocarpine, carbachol other parasympathomimetics | Any |
| Prostaglandin derivatives (latanoprost) | Any |
| Sulfamethazine/trimethoprim (immune-mediated) | Dogs |
| Endotoxemia from any systemic source | Any |
| Infectious keratitis with bacterial toxin production | Any |
| Radiation therapy | Any |
| TRAUMA | |
| Blunt or penetrating injuries | Any |
| Corneal foreign bodies | Any |
| REFLEX UVEITIS | |
| Ulcerative keratitis of any cause | Any |
| Deep necrotizing or nonnecrotizing scleritis | Dog |
| Episcleritis | Dog |
MCF, Malignant catarrhal fever.
* Neosporum caninum has been found responsible for some cases of dogs previously diagnosed with T. gondii infection. The clinical significance is undetermined.
Numerous uveitis classification schemes have been proposed, including those based on the tissues affected (anterior uveitis, posterior uveitis, panuveitis), on the presumed histologic nature of the disorder (suppurative, nonsuppurative, granulomatous, nongranulomatous), on whether the cause starts inside the eye or from its surface (endogenous versus exogenous), and on a specific etiology (see Table 11-4). Although each of these schemes has its own advantages and disadvantages, classification into granulomatous or nongranulomatous and then by specific etiology is probably the most useful method in a clinical setting, because it also helps guide specific therapy (Table 11-5). This scheme, however, is plagued by the presence of a large percentage of patients having idiopathic uveitis in which the cause remains obscure and therapy can be only nonspecific and directed at controlling inflammation and preventing further damage to the eye. Presumably, most of these cases are immune-mediated or involve microorganisms that are not yet recognized as pathogenic. It is hoped that over time the percentage of patients with idiopathic uveitis will decline as our understanding of the causes of this disorder improves.
Table 11-5 Classification Criteria for Anterior Uveitis
| NONGRANULOMATOUS | GRANULOMATOUS |
|---|---|
| Acute onset | Gradual onset |
| Short course | Chronic or recurrent |
| No keratic precipitates | Keratic precipitates/greasy exudate on lens surface |
| No synechiae | Posterior synechiae |
| No iris nodules | Iris nodules may be present |
| Primarily anterior uveitis | Posterior uveitis may also be present |
These criteria are useful but not absolute and are interpreted along with other clinical signs.
Although classification as granulomatous or nongranulomatous uveitis is based on a histologic classification scheme, the criteria in Table 11-5 can also be used to make reasonable clinical inferences about the histologic nature of the inflammation and to allow for prioritization of the diagnostic tests to be performed. Most cases of granulomatous uveitis are associated with microorganism or foreign material stimulation of a chronic immune response, whereas nongranulomatous uveitis is often associated with physical, toxic, or allergic causes. After determining whether a specific animal has granulomatous or nongranulomatous uveitis, the clinician should consider specific tests to try to determine the exact cause (e.g., serum titer measurement for Toxoplasma). In general the following specific categories of uveitis should be considered:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree