W. David Wilson, Nicola Pusterla, Paul J. Plummer, Victor S. Cortese, Consulting Editors W. David Wilson and Nicola Pusterla, Consulting Editors Programs for controlling infectious diseases are important components of management practices directed toward maximizing the health, productivity, and performance of horses.1 Infectious disease in an individual horse or outbreaks of infection in a group occurs when horses experience challenge with an infectious agent at a dose sufficient to overcome resistance acquired through previous natural exposure to the disease or through vaccination. For this reason, programs for controlling infectious diseases should have the following three goals: 1. To reduce exposure to infectious agents in the horses’ environment 2. To minimize factors which diminish resistance The incidence of infectious disease in horse populations tends to rise with an increased number and stocking density of susceptible horses at a facility, with movement of horses on and off the facility, and with favorable external environmental and management influences. Other factors that influence the risk of acquiring infection and developing disease include the age, type, breed, gender, and use of the animals; geographic, climatic, and other environmental factors; facilities’ layout and management practices; and history of exposure to or vaccination against individual diseases. The conditions on breeding farms, in performance and show horse barns, and at racetracks are ideal for introduction and transmission of infectious diseases, particularly those of the respiratory tract. On breeding farms, the introduction and commingling of horses of various ages and origins and the high proportion of young susceptible horses and pregnant mares create a situation that poses special problems and demonstrates some important considerations in the practice of disease control. The risk of acquiring infection can be reduced by maintaining distinct groups by age and function. Resident mares and foals should be kept separate from weanlings, yearlings, horses in training, and visiting mares. Visiting mares and other horses entering the farm should have a negative test result for equine infectious anemia (EIA), either by ELISA or the Coggins AGID test, and should be appropriately vaccinated and dewormed before arrival. They should be received and maintained in barns and paddocks separate from the resident farm population. Preferably, a specific group of caretakers should attend to incoming horses, and footbaths, separate equipment, and a clean change of coveralls and boots should be used. New arrivals should be quarantined for 30 days and monitored for signs of contagious disease. The rectal temperature should be recorded at least once daily, and any prophylactic procedures not done before arrival should be performed. Foaling mares being sent to a distant breeding farm for breeding should be transported 6 to 8 weeks before foaling; this permits timely exposure to resident pathogens at the destination farm, which allows the mare’s immune system to mount a response and concentrate antibodies in the colostrum to improve passive protection of the foal. Mares being shipped short distances for breeding can be transported during estrus and returned to the farm on the same day to reduce the risk of the foal acquiring infection. Regardless of the type of equine facility, any horse that becomes ill with a possibly contagious disease should be isolated, preferably in an air space separate from the remainder of the herd, for at least 10 days beyond complete abatement of clinical signs. Separate equipment should be used, and if a separate group of caretakers is unavailable for these animals, workers should always complete their work with healthy horses before handling sick horses. Caretakers should wash their hands and boots thoroughly between horses and wear different outer clothing or coveralls. Stalls that have housed sick horses should be cleaned thoroughly, disinfected, allowed to dry, and left empty as long as possible. This approach is particularly important in dealing with organisms such as Streptococcus equi that can survive in a protected moist environment for several weeks.2 In most equine enterprises, vaccination is important to the overall management program for controlling infectious diseases. No “standard” vaccination program can be recommended for all horses; each situation must be evaluated individually by weighing the risk of acquiring infection and the medical and economic consequences of infection against the cost and expected efficacy of the product or products being considered for inclusion in the program, and their potential for inducing adverse reactions. Cost should include expenses incurred and money lost during the time the horses are out of competition, labor and medication expenses if the animals develop clinical disease and require treatment, and the expenses in time, labor, and vaccines required for proper immunization. The client’s expectations should be realistic, and the veterinarian should explain the following points carefully: • Vaccination minimizes the risk of infection but does not prevent disease in all circumstances. • Horses in a population are not all protected equally nor for an equal duration after vaccination. A properly administered licensed product should not be assumed to provide absolute effective protection during any given field epidemic. Copies of the vaccination and health maintenance records should accompany each horse leaving the facility for sales, training, or breeding. Similarly, owners of equine facilities should establish prerequisites for vaccination of all horses entering the facility and request that copies of the vaccination records accompany those horses. Client expectations and the goals of disease control programs vary considerably. In performance horses, the goal generally is to minimize time spent out of training and thereby maximize earning potential. In this case, an enforced period of rest owing to infectious disease has much more profound economic consequences than a similar recommendation for a barren broodmare or backyard horse. On the other hand, many owners of backyard horses diligently vaccinate against even low-risk diseases, despite the expense involved, to keep their horses healthy. Only federally licensed vaccines should be used, and strict attention must be paid to the manufacturer’s recommendations for storage, handling, and routes of administration to maximize the product’s efficacy and safety. However, research or clinical experience may support alternate protocols for vaccination that will improve the vaccine’s efficacy without increasing adverse effects. The length of time necessary to induce a protective immune response should be considered in relation to expected exposure. When inactivated (killed) vaccines are administered by intramuscular (IM) injection, for example, optimal protection is generally not achieved until 2 to 3 weeks after completion of the primary series, or 1 or more weeks after administration of a booster dose. Inactivated vaccines administered IM generally induce a greater serologic response when an initial series of 3 doses is given rather than the 2-dose series recommended by most vaccine manufacturers. Whereas a 3- to 4-week interval between the first and second doses of vaccine is generally appropriate, a longer interval of 3 to 5 months between the second and third doses appears to optimize priming of the immune system and protection. The primary role of authorities charged with licensing vaccines in North America has traditionally been to ensure vaccine purity and safety, with less emphasis placed on documentation of efficacy.3–5 Consequently, little published information was available in the past documenting the efficacy of most vaccines licensed in North America. The situation has improved substantially in recent years, to the extent that published efficacy data are available for almost all equine vaccines licensed in North America since 1999. Field experience and some experimental evidence suggest that the efficacy of vaccines directed against different diseases varies considerably and that efficacy also varies among the vaccines from different manufacturers directed against the same disease.6–8 Vaccination is unlikely to confer protection more durable than that produced by recovery from natural disease, especially when the route of vaccination (usually IM) is different from the route of natural infection; this is because vaccines frequently do not evoke the full array of protective immune responses induced by natural infection.9,10 For example, the efficacy and durability of protection induced by parenteral vaccines against respiratory tract pathogens are frequently questioned.4,5,10 In part this reflects the fact that parenterally administered vaccines are generally poor inducers of the local mucosal immune responses that are important for effective protection against infection of the respiratory tract.4,9,10 In addition, immunity achieved after natural infection with some respiratory tract pathogens is short lived. The primary goals of vaccination programs for broodmares are (1) prevention of diseases that pose a risk to the mare or her fetus, and (2) maximizing the level of colostral antibodies that will be passively absorbed by the neonatal foal after nursing, thus providing it with protection against diseases that pose a risk during the first few months of life. Additional considerations in selecting vaccines for use in pregnant mares include (3) safety to the mare and fetus, (4) the potential for interference between multiple vaccines administered simultaneously, and (5) the influence of pregnancy on vaccine responses. Broodmares are at risk of exposure to the same diseases as performance and pleasure horses, so they should be regularly vaccinated against all core and specific risk-based diseases according to published recommendations. The high horse traffic and high concentration of foals and young horses that typify many breeding farms contribute to a high risk of exposure to contagious respiratory diseases, including equine herpesvirus (EHV)-4, EHV-1, influenza, and strangles. Inclusion of influenza in vaccination protocols for broodmares is routinely recommended, and addition of EHV-4 and strangles vaccines is frequently recommended when conditions of significant risk are anticipated. Vaccination of mares against equine viral arteritis (EVA) before breeding may be indicated when they are to be bred to a known or suspected EVA carrier stallion, either by natural cover or by artificial insemination. Whereas protection of the broodmare, fetus, or herdmates against the abortifacient effects of EHV-1 or EVA is the primary goal underlying inclusion of these antigens in vaccination protocols for broodmares, the goal of protecting the foal features at least as prominently in the rationale for vaccinating mares against tetanus, West Nile virus (WNV), eastern equine encephalitis (EEE), western equine encephalitis (WEE), rabies, influenza, EHV-4, and strangles. Inclusion of rotavirus and botulism vaccines in protocols for pregnant mares is directed almost exclusively at protecting the young foal against these diseases. Maintaining consistent broodmare vaccination protocols, which typically include administration of booster doses of vaccines during the last 2 months of gestation, will not only protect the mare but also maximize the likelihood that a uniformly high level of maternally derived antibody (MDA) transfer and passive protection will be achieved within the foal crop. This is particularly important for diseases that pose a risk to the foal during the first few weeks of life. Whereas intranasally administered vaccines may afford good protection to the mare, they are typically less effective than parenterally administered inactivated vaccines in stimulating high levels of circulating immunoglobulin (Ig)G, the isotype that is passively transferred to the foal in highest concentration. Parenterally administered vaccines are preferred over intranasally administered vaccines for vaccination of mares during late gestation. Consideration of vaccine safety in broodmares must take into account risks to the pregnancy and safety to the fetus. Potential adverse effects of vaccines on pregnancy are difficult to document even when large numbers of mares are used, unless obvious problems occur. Because fetal organogenesis occurs early in gestation, and this period is also characterized by substantial embryonic loss, even in normal mares it is sound practice to avoid administering vaccines to mares during the first 60 days of gestation unless conditions of imminent risk prevail. Few vaccines carry specific label recommendations for use in pregnant mares and little published data exists to specifically document the safety of equine vaccines during pregnancy. Licensed vaccines that carry label recommendations for use in pregnant mares include two inactivated EHV-1 vaccines (Pneumabort-K+1b [Zoetis] and Prodigy [Merck Animal Health]) marketed for use in pregnant mares as an aid in prevention of EHV-1 abortion, the Calvenza line of inactivated influenza (Calvenza-03 EIV [equine influenza virus]), EHV-1 (Calvenza EHV), and influenza/EHV-1 combination (Calvenza-03 EIV/EHV) vaccines from Boehringer Ingelheim, one inactivated Potomac horse fever vaccine (Equine Potomavac [Merial]), and one vaccine licensed for prevention of type B botulism in foals (BotVaxB [Neogen]). One conditionally licensed vaccine (Equine Rotavirus Vaccine [Zoetis]) for prevention of rotavirus infection in foals is labeled for use in pregnant mares. While not specifically labeled for administration during pregnancy, widespread use in practice over many years has failed to document that any of the inactivated vaccines currently marketed for use in horses pose an unacceptable risk to pregnant mares. Therefore, pregnant mares are routinely vaccinated with inactivated vaccines directed against tetanus, EEE, WEE, WNV, influenza, EHV-4, strangles and, to a lesser extent, Potomac horse fever (PHF), rabies, and Venezuelan equine encephalitis (VEE). Similarly, adverse impacts on pregnancy have not been documented for modified live intranasally administered strangles (Pinnacle I.N. [Zoetis]) and influenza (Flu-Avert [Merck Animal Health]) vaccines or the modified live parenterally administered EHV-1 vaccine (Rhinomune [Boehringer Ingelheim]). Safety of the recombinant WNV and influenza vaccines (Recombitek [Merial]) should not be a significant concern because the modified live canarypox vector lacks the ability to infect mammalian cells. In addition, the equivalent canarypox-vectored influenza vaccine (ProteqFlu [Merial]) marketed in the United Kingdom is labeled for use during pregnancy. Although the Flavivirus chimera WNV vaccine (PreveNile [Merck Animal Health]) was not specifically labeled for use during pregnancy while it was on the market, more than 300 pregnant mares were vaccinated during safety trials for licensing, without apparent adverse effects on the conceptus. In contrast, modified live virus (MLV) VEE vaccines and live anthrax spore vaccines should not be used in pregnant mares. Protection of mares against the potential abortigenic effects of EVA infection is best accomplished by completing the primary immunization series before the mare enters the broodmare band and by administering subsequent boosters during the open period before rebreeding.11 The practice of booster vaccinating mares against multiple diseases to maximize colostral transfer of antibodies to the foal results in the typical broodmare receiving multiple doses of many vaccine antigens and adjuvants during her lifetime. In addition to stimulating high levels of antibody against a range of antigens, this practice may also predispose these mares to a higher rate of local and systemic adverse reactions, an issue that not only warrants further investigation but may force horse owners and veterinarians to carefully consider strategies for revaccination. The possibility that “competition” between multiple antigens will compromise the response to some or all of the administered antigens should be considered. When administration of multiple vaccines late in gestation is indicated, it is good practice to administer no more than 4 antigens at one time and to allow an interval of 3 to 4 weeks between administering different vaccines. It is widely assumed that pregnant mares are fully capable of mounting appropriate cellular and humoral immune responses to vaccines, but this issue has received little research attention. Mares that have been primed before breeding appear to mount appropriate anamnestic responses to vaccines, but preliminary data suggest that the humoral response to primary vaccination with several inactivated vaccines, including West Nile Virus and rabies, may be downregulated during gestation, resulting in failure of some vaccinated mares to passively transfer specific antibodies to the foal via colostrum. Maternally derived antibodies (MDAs) and perhaps other immune effectors (e.g., lymphocytes) that are concentrated in colostrum and are passively transferred to the foal play a crucial role in defense against pathogens encountered during the first few months of life while endogenous immune function continues to mature. Passive transfer of MDAs should therefore be exploited in immunization programs for foals by consistently administering booster doses of selected vaccines to mares 4 to 8 weeks before foaling and by ensuring that foals ingest adequate amounts of high-quality colostrum within 24 hours of birth. Besides passively protecting the foal, MDAs may also exert a profound inhibitory effect on the active immune response of the foal to antigens, including those contained in vaccines. This phenomenon is known as maternal antibody interference. Several studies reported during the 1990s brought this issue into focus by demonstrating that foals younger than 6 months of age consistently failed to mount serologic responses to inactivated influenza vaccines.12–18 Of potentially greater concern was the finding that a high proportion of foals vaccinated under the cover of MDAs not only failed to seroconvert in response to the recommended primary series of two or three doses of influenza vaccine, but many also failed to respond to multiple additional doses administered during the next year, suggesting induction of a potentially detrimental immunotolerance-like phenomenon.15,16,19 Our studies confirmed an apparent lack of response of foals to multiple doses of inactivated influenza vaccines when the hemagglutination inhibition (HI) test was used to detect serologic responses, but responses were detected when the same samples were assayed using sensitive isotype-specific enzyme-linked immunosorbent assay (ELISA). Rather than representing true tolerance, it appears MDAs may cause misdirection of the immune response away from the more important virus neutralizing IgGa and IgGb subisotypes in favor of the less effective IgG(T) sub-isotype of IgG.12 Subsequent studies in which titers of total rather than antigen-specific IgG subisotypes were determined documented that the age-related increase in concentrations of IgGb lagged significantly behind increases in concentrations of other isotypes and remained below adult levels beyond 6 months of age.20 Maternal antibody interference has now been documented to be a significant issue for many other antigens, including tetanus, EEE, WEE, EHV types 1 and 4, contained in vaccines administered to foals.12,21–25 Even low levels of antibody below those detectable by many routine serologic tests and below those thought to be protective can completely block the serologic response to some vaccines, resulting in a potentially prolonged period of susceptibility before the foal is capable of responding appropriately to vaccines.24 These findings also indicate that it is not typically feasible to serologically test samples from foals to predict whether they will respond to particular vaccines. We now recommend that primary immunization with most vaccines containing inactivated antigens should be delayed until foals are 6 months of age or older, and with the exception of rabies vaccine, three doses of vaccine should be included in the primary series rather the two doses routinely recommended by vaccine manufacturers. Typically, the third dose stimulates a serologic response of greater magnitude and durability than two doses and may also contribute to a higher “set-point” for the response to subsequent booster doses.12,24,26,27 In contrast to the results cited, maternal antibodies do not appear to exert a marked inhibitory effect on the immune response of foals to the inactivated, live recombinant, live chimera, or DNA West Nile virus vaccines, thereby permitting antibody-positive foals as young as 3 months of age to be immunized successfully.26,28,29 Similarly, the canarypox-vectored recombinant influenza vaccine has been shown to efficiently prime foals in the presence of MDA.30 Study results should be interpreted with caution because only humoral responses are typically assessed in MDA interference studies, and infectious challenge is not performed to confirm that lack of serologic response equates to lack of protection. Lack of a serologic response may correlate well with lack of protection for some diseases and some vaccines, whereas for others this may not be the case. In contrast, the presence of a serologic response may not correlate well with protection, as is frequently the case for respiratory tract pathogens. Because many commercially available vaccines are inactivated, adjuvanted, and administered by IM injection, they have limited potential to stimulate cellular and mucosal responses, so serologic responses induced by these vaccines likely correlate well with their potential to induce protection. In turn, MDA interference with serologic responses to inactivated vaccines likely equates to failure to induce protection. In contrast, failure to detect a serologic response to a modified live, vectored, DNA, or mucosally administered vaccine may not equate to lack of protection, because vaccines of these types induce a broader array of systemic and local responses that may not be affected by MDAs. If MDA interference were not an issue, the approach to vaccination of foals would be greatly simplified because primary vaccination against all of the important diseases could be completed before MDAs had declined to non-protective levels. In effect, the “window of susceptibility” would be eliminated. In reality, an attainable goal is to maximize the beneficial effects of MDAs while minimizing their negative impact on primary immunization. To best meet this goal it is necessary to decide which one (or both) of the following is the primary focus: (1) to protect the foal and weanling against specific high-risk infectious diseases that affect this age group and have the potential to cause significant disease, either directly or by predisposing to other secondary infections, or (2) to initiate primary immunization to protect against disease later in life. Assessing risk takes into account both the likelihood the foal will become infected and the risk of serious sequelae or death if the horse does become infected and develop disease. If the disease affects the foal early in life, such as is the case with rotavirus (RV) infection, there is usually insufficient time to induce a protective immune response by actively immunizing the foal. Under these circumstances, the approach should be to maximize the degree of protection passively transferred from the dam via colostrum. Other diseases like rabies can affect horses of all ages, but the risk of acquiring infection is generally low. Diseases of moderate to high risk to young foals but low risk to adults include RV infection (on certain breeding farms in certain years) and, in geographic areas such as Kentucky and some other Eastern states, type B botulism. For these diseases, the following approach is appropriate: Diseases of moderate to high risk for weanlings and older horses but lower risk to young foals born to vaccinated mares include EHV-4, EHV-1, strangles, influenza, tetanus, EEE, and WNV infection. For these diseases, the following approach is appropriate: Diseases of low risk to foals in most circumstances include rabies, PHF, WEE, and EVA. For these diseases, the following approach is appropriate: Though uncommon, the possibility always exists for adverse reactions (including anaphylaxis) associated with vaccine administration, so vaccines should be administered by or under the direct supervision of a veterinarian. Adverse reactions should be reported to the vaccine’s manufacturer and the U.S. Department of Agriculture (USDA) (1-800-752-6255) or the U.S. Pharmacopeia (USP) Veterinary Practitioners Reporting Program (forms may be obtained or reports submitted by calling the USP at 1-800-487-7776). Anaphylaxis constitutes a life-threatening emergency requiring prompt treatment with epinephrine (0.01 to 0.02 mg/kg; equivalent to 5 to 10 mL of a 1 : 1000 dilution IM for a 450-kg horse). Repeated doses of epinephrine can be administered at 15-minute intervals if necessary. It has recently been shown that horses vaccinated with viral vaccines can develop IgE responses to non-target antigens, including bovine serum albumin (BSA), suggesting that subsequent administration of another viral vaccine containing the same component could elicit an adverse response, including anaphylaxis.31 Local irritant tissue reactions occur more frequently, particularly when polyvalent combination vaccines and injectable strangles vaccines are used. These reactions usually are self-limiting, but resolution can be promoted by parenteral or oral (PO) administration of nonsteroidal antiinflammatory drugs (NSAIDs), topical application of warm compresses or the cutaneously absorbed NSAID diclofenac (Surpass [IDEXX Pharmaceuticals, Greensboro, N.C.]), and gentle exercise. Significant reactions in the neck muscles may make the horse reluctant to lower or raise its head; feed and water buckets should be positioned accordingly. Occurrence of externally visible local reactions can be reduced by administration of the vaccine deep in the semimembranosus and semitendinosus muscles of the hind-leg rather than in the neck, and by allowing the horse to exercise after vaccination. Horses that repeatedly react to polyvalent vaccines may benefit from NSAID administration before vaccination, administration of the individual antigenic components separately in different sites, use of a different brand of vaccine, use of a vaccine that can be administered by a route other than IM, or use of a vaccine that contains a different adjuvant or no adjuvant at all. Some horses develop transient self-limiting systemic signs that may include fever, anorexia, lethargy, colic, diarrhea, tachycardia, and congested mucous membranes after IM administration of vaccines. Systemic signs are perhaps more common with certain vaccines but can be associated with any vaccine.32,33 In addition, inactivated Immune Stimulating Complex (ISCOM) and live recombinant vectored tetanus and influenza combination vaccines have been shown to elicit a prominent acute phase inflammatory response of several days’ duration in vaccinated horses.34 A similar response likely occurs with other vaccines. It is therefore inadvisable to give horses any injectable vaccine within 2 weeks before a show, performance event, sale, or prolonged transportation. It may also be beneficial to minimize environmental dust when vaccinating horses known to have allergic airway disease or hypersensitivity.32 If unacceptable reactions occur repeatedly, the need for continued annual or more frequent revaccination against individual antigens should be carefully reevaluated, taking into account risk of disease balanced against the risk of an adverse reaction. Many horses that experience adverse reactions have received many doses of many vaccine antigens, repeated over many years. In this situation, the vaccination protocol should be “pared down” so only the most essential antigens are administered and the maximum possible interval between boosters is employed. For diseases like rabies and tetanus for which resistance can reasonably be correlated with circulating antibody titer, one possible approach to define the maximum or optimal interval between booster doses would be to measure the antibody titer. Unfortunately, this approach is currently limited by the paucity of laboratories that offer this type of testing on a routine basis, inexpensively, and with a short turnaround time. Introduction of commercially available ELISA testing for antibodies to the SeM protein of Streptococcus equi subsp. equi (Equine Biodiagnostics-IDEXX, Lexington, Ky.) and neutralizing antibody testing for WNV virus (Cornell University, Colorado State University, the University of Florida, and the USDA Animal and Plant Health Inspection Service [APHIS] National Veterinary Services Laboratory) in recent years has made it possible to refine vaccination protocols for these diseases in horses that experience adverse reactions to vaccination. Testing for rabies antibodies is available through Kansas State University, and testing for antibodies to other pathogens may be available through state diagnostic laboratories. Fully licensed vaccines are now available in North America as aids to the prevention of tetanus, viral encephalomyelitis (EEE, WEE, VEE), WNV infection, influenza, EHV-1 and EHV-4 infection, strangles, rabies, EVA, PHF, and type B botulism. In addition, conditionally licensed vaccines are available to immunize horses against rotavirus and equine rhinitis A virus (ERAV). Tetanus, rabies, and viral encephalomyelitis caused by EEE, WEE, and WNV pose a threat to horses in all geographic areas and are therefore considered to be core diseases against which all horses in North America should be vaccinated. The abortigenic potential of EHV-1 warrants inclusion of this disease in the core for all pregnant broodmares. Although influenza is not routinely included as a core disease, vaccination against this highly contagious respiratory tract infection is strongly recommended for all horses likely to be co-located with horses from other facilities during transportation or at sales, shows, trail rides, races, or other events. The remaining diseases for which vaccines are available are considered “non-core” or “risk based.” Indications for use of vaccines against these diseases will be discussed in relevant sections that follow later in this chapter. Table 48-1 details the types of vaccines licensed for use in horses in the United States. Tables 48-2 through 48-4 provide general guidelines for use of the most frequently indicated equine vaccines in foals, weanlings, yearlings, and adult horses under various management conditions and in various geographic locations. TABLE 48-2 Guidelines for Vaccination of Adult Horses, Excluding Broodmares, Against Core and Non-Core (Risk-Based) Diseases • Horses residing in endemic areas with a prolonged vector season • Juvenile horses (<5 years of age) • Geriatric horses (>15 years of age) * Core vaccines protect against diseases that are endemic to a region, are virulent or highly contagious, pose a risk of severe or fatal disease, have potential public health significance, and/or are required by law. Core vaccines have clearly demonstrable efficacy and have a sufficiently high level of patient benefit and low level of risk to justify their use in all equids in North America. † Non-core (risk-based) vaccines are selected for use based on assessment of risk performed by, or in consultation with, a licensed veterinarian. Use of non-core vaccines will vary between individuals, populations, and/or geographic regions. Modified with permission from recommendations developed by the AAEP Infectious Disease Committee and posted on the AAEP website (aaep.org). TABLE 48-3 Guidelines for Vaccination of Broodmares Against Core and Non-Core (Risk-Based) Diseases * Core vaccines protect against diseases that are endemic to a region, are virulent or highly contagious, pose a risk of severe or fatal disease, have potential public health significance, and/or are required by law. Core vaccines have clearly demonstrable efficacy and have a sufficiently high level of patient benefit and low level of risk to justify their use in all equids in North America. † Non-core (risk-based) vaccines are selected for use based on assessment of risk performed by, or in consultation with, a licensed veterinarian. Use of non-core vaccines will vary between individuals, populations, and/or geographic regions. Modified with permission from recommendations developed by the AAEP Infectious Disease Committee and posted on the AAEP website (aaep.org). TABLE 48-4 Guidelines for Vaccination of Foals, Weanlings, and Yearlings Against Core and Non-Core (Risk-Based) Diseases * Core vaccines protect against diseases that are endemic to a region, are virulent or highly contagious, pose a risk of severe or fatal disease, have potential public health significance, and/or are required by law. Core vaccines have clearly demonstrable efficacy and have a sufficiently high level of patient benefit and low level of risk to justify their use in all equids in North America. † Non-core (risk-based) vaccines are selected for use based on assessment of risk performed by, or in consultation with, a licensed veterinarian. Use of non-core vaccines will vary between individuals, populations, and/or geographic regions. Modified with permission from recommendations developed by the AAEP Infectious Disease Committee and posted on the AAEP website (aaep.org). All horses are at risk for developing tetanus, an often fatal disease caused by a potent neurotoxin elaborated by the anaerobic spore-forming bacterium Clostridium tetani. Infection of tissues typically occurs via puncture wounds (particularly those involving the foot or muscle), open lacerations, surgical incisions, exposed tissues like the umbilicus of foals, and the reproductive tract of the postpartum mare (especially in the event of trauma or retained placenta). C. tetani is present in the intestinal tract and feces of horses, other animals, and human beings, and spores are abundant as well as ubiquitous in soil. Spores of C. tetani survive in the environment for many years, resulting in an ever-present risk of exposure of horses and people on equine facilities. Because tetanus is expensive to treat and has a high mortality rate, all horses should be actively immunized using tetanus toxoid as part of the core vaccination program. Active immunization reduces the need to administer tetanus antitoxin, the use of which is associated with risk of inducing potentially fatal serum hepatitis. Protection against tetanus is mediated by circulating antibodies; toxin binding inhibition (ToBi) antibody titers of greater than 0.2 IU/mL are considered to be protective in the horse.27,35 The many available vaccines are formalin-inactivated, adjuvanted toxoids that are inexpensive, safe, and potent antigens that induce an excellent serologic response and solid long-lasting immunity when administered according to manufacturer recommendations. Primary immunization involves administration of 2 doses of toxoid at 3- to 6-week intervals. Titers of specific antibody increase to protective levels within 14 days after administration of the second dose in the primary series and, in adult horses, persist at detectable levels for 12 months or longer, depending on the adjuvant system used in the vaccine.27,35–37 A recent study documented substantial differences between currently licensed combination tetanus-encephalomyelitis vaccines with regard to the magnitude of the vaccine-induced tetanus specific IgGb and IgG(T) antibody responses.8 The vaccine containing a Carbopol adjuvant induced substantially higher antibody titers than those containing either saponin or squaline combined with surfactants.8 Revaccination once annually is recommended. No published challenge studies are available to document the speed of onset or duration of protection induced by tetanus toxoid preparations currently licensed in North America. Conclusions regarding their efficacy are therefore based on the serologic response obtained in horses and laboratory animals and on field experience. However, a challenge study conducted in Europe almost 60 years ago found that horses were resistant to challenge 8 days after receiving a single injection of tetanus toxoid, before antibody could be detected in their serum.38 A second study demonstrated that a series of 3 doses of tetanus toxoid induced protection lasting for at least 8 years, and perhaps for life, even when antibodies could no longer be detected.35 A recent study in Europe documented that protective levels of antibody (>0.2 IU/mL as measured by tetanus toxin-binding ELISA) persist for at least 18 months after revaccination of horses previously primed with a 2-dose series of a tetanus toxoid/influenza ISCOM vaccine.39 In contrast, tetanus has been documented in vaccinated horses in North America,40 although survival was strongly associated with previous vaccination. Thus it would not be prudent to recommend extension of the annual interval for revaccination with tetanus toxoid products available in North America, pending publication of data documenting duration of immunity (DOI). Vaccinated horses that sustain a wound or undergo surgery more than 6 months after receiving their previous tetanus booster should be revaccinated with tetanus toxoid immediately at the time of injury or surgery. Annual revaccination of pregnant mares should be completed 4 to 8 weeks before foaling to protect the mare if she sustains foaling-induced trauma or retained placenta and to enhance concentrations of specific immunoglobulins in colostrum. Colostrum-derived antibodies significantly interfere with the immune response of foals vaccinated with tetanus toxoid until they are about 6 months old.12,36 Primary vaccination of foals that have received appropriate transfer of colostral antibodies from a vaccinated mare should include 3 doses of tetanus toxoid beginning at age 6 months or older. Optimally, the interval between the first 2 doses of vaccine should be approximately 4 weeks, and the interval between the second and third doses should be 3 to 5 months. The 3-dose primary series is recommended for foals, because a high proportion of foals fail to seroconvert in response to 2 doses of tetanus toxoid regardless of whether maternal antibodies are detectable at administration of the first dose.12,36 For foals born to non-immune mares, this initial 3-dose series can start at 1 to 4 months of age. Tetanus antitoxin is produced by hyperimmunization of donor horses with tetanus toxoid. Administration of 1 vial of antitoxin (1500 IU) to nonvaccinated horses induces immediate passive protection that lasts not more than 3 weeks.36 More prolonged protection may be accomplished with higher doses. In addition to the use of high doses of tetanus antitoxin to treat tetanus, indications frequently cited include administration to newborn foals born to unvaccinated mares and to unvaccinated horses that sustain an injury. In these cases the concurrent administration of tetanus antitoxin and tetanus toxoid at different sites using separate syringes has been advocated, followed by administration of additional doses of toxoid at 4- to 6-week intervals to complete the primary series.41 Because a small but significant number of horses experience serum sickness and fatal hepatic failure (serum hepatitis) several weeks after receiving tetanus antitoxin,42,43 a preferred approach to the unvaccinated horse that sustains a puncture or deep laceration is to thoroughly clean and débride the wound, initiate active immunization by administering tetanus toxoid, and institute a course of antimicrobial treatment with penicillin or alternate antimicrobial that is active against C. tetani. The equine encephalomyelitis viruses (EEE, WEE, and VEE) belong to the Alphavirus genus of the family Togaviridae. They are transmitted by mosquitoes (and infrequently by other bloodsucking insects) to horses from wild birds or rodents that serve as natural reservoirs for these viruses. Risk of exposure and geographic distribution of the encephalomyelitis viruses vary by season and from year to year with changes in distribution of insect vectors and wildlife reservoirs. The distribution of EEE has historically been restricted to the eastern, southeastern, and some southern states, with recent northward encroachment. WEE has caused minimal disease in horses in North America during the past 3 decades, but the virus continues to be detected in mosquitoes and birds throughout the western states. In the past, outbreaks of WEE have been recorded in the western and midwestern states, with sporadic cases in the northeastern and southeastern United States. Because EEE, WEE, or both are endemic in most areas of North America, vaccination against these diseases should be part of the core vaccination program for all horses. VEE is a reportable foreign animal disease. Epidemics of VEE occur when the virus undergoes genetic change and develops greater virulence for avian and mammalian hosts. These viral variants are able to multiply to high levels in the horse, and then the horse becomes a reservoir for infection in these outbreaks. VEE occurs in South and Central America but has not been diagnosed in the United States or Mexico for many years; routine vaccination of horses in these regions against VEE is not recommended at this time unless transportation to endemic areas is planned. Available vaccines are formalin inactivated, adjuvanted, bivalent or trivalent, whole-virus products containing EEE, WEE, and VEE, typically in combination products containing other antigens such as tetanus, influenza, WNV, or EHV. Although correlates for protection against EEE, WEE, and VEE are not well established, circulating antibodies are assumed to be important because infection is acquired by vascular injection (mosquito bites), and current inactivated vaccines appear to have good efficacy.44,45 A study evaluating the serologic response of horses to commercial encephalomyelitis-tetanus combination vaccines showed that the EEE neutralizing antibody responses to Encevac T (Merck Animal Health, Carbopol adjuvant) and Equiloid Innovator (Zoetis, squaline and surfactant adjuvant) were of greater magnitude and persistence than responses to Cephalovac EWT (Boehringer Ingelheim, saponin adjuvant).8 However, no comparative randomized challenge studies have been performed using these vaccines to document whether differences in serologic responses equate to differences in efficacy. Early testing of bivalent (EEE/WEE) vaccines was performed by intracranial challenge with either EEE or WEE; the formalin inactivated preparations demonstrated 100% protection. Primary immunization of unvaccinated adult horses is accomplished by administering 2 doses of inactivated vaccine 3 to 6 weeks apart. In areas where EEE is not a threat and mosquito vectors are active for less than 6 months of the year, annual revaccination in the spring, before the peak insect vector season, is recommended. In areas like the Gulf States where EEE is endemic and mosquitoes are active virtually year-round, many veterinarians prefer to revaccinate horses semiannually to ensure more uniform protection throughout the year. Inactivated encephalomyelitis vaccines are considered safe for use during pregnancy, so booster vaccination of pregnant mares 4 to 8 weeks before foaling is routinely recommended to enhance colostral concentrations of specific immunoglobulins. Neutralizing antibodies to WEE and EEE are transferred passively to foals through colostrum and decline with an estimated half-life of 33 and 20 days, respectively. MDAs appear to confer protection and are detectable in the serum of many foals from vaccinated mares for at least 3 months and up to 7 months, depending on the post-nursing titer.23,46–48 Several studies have shown that MDAs exert a profound inhibitory effect on the ability of foals to mount serologic responses to inactivated bivalent WEE/EEE vaccines, which likely accounts for some of the reported cases of vaccine failure and resultant clinical EEE in vaccinated horses, particularly those younger than 2 years of age.19,23,24,46,47 Studies have shown that 3-month-old foals born to immune mares consistently failed to mount a serologic response to 2 doses of inactivated bivalent WEE/EEE vaccine, and the majority had not responded even after administration of a third dose.24,26 Whereas many 6-month-old foals failed to seroconvert after administration of 2 doses of vaccine, most responded following administration of a third dose.24 Based on these data, inclusion of a third dose in the primary series, 2 to 5 months after administration of the second dose, is strongly recommended for primary immunization of foals and yearlings. WEE has a lower mortality rate than EEE, and prevalence of WEE in many western states is sufficiently low that the risk of foals acquiring infection during their first year of life is also low. Therefore, primary vaccination of foals of vaccinated mares in areas where mosquitoes die off in the winter and the risk of infection is low is best completed when foals are 5 to 6 months of age or older in order to minimize the potential for MDA interference. Because foals born in the late spring and summer months are still less than 6 months of age by the time the mosquito season comes to an end in many regions, primary vaccination of these foals can be delayed until the spring of the yearling year. In contrast, EEE is a highly fatal disease that poses a significant risk to foals during their first year of life, particularly in the Gulf States where competent vectors are present year-round.19,47,49 Most veterinarians in these regions recommend commencing primary vaccination of foals at 3 to 4 months of age using a 3-dose primary series, followed by a fourth dose before the onset of the next mosquito season and semiannual boosters thereafter to maximize the chances of overcoming the inhibitory effects of MDAs and inducing protection.47
Use of Biologics in the Prevention of Infectious Diseases

Current Concepts in Equine Vaccination and Infectious Disease Control
General Considerations
Considerations for Use of Vaccines in Broodmares
Protecting the Mare Against Diseases That Pose a Risk to the Mare or Her Fetus.
Maximizing Maternally Derived Antibody Transfer.
Vaccine Safety in Broodmares.
Potential Interference between Multiple Antigens Administered Concurrently.
Influence of Pregnancy on Vaccine Responses.
Vaccination of Foals and Influence of Maternal Antibodies on Vaccine Responses
Diseases of Moderate to High Risk to Young Foals but Low Risk to Adults
Diseases of Moderate to High Risk for Weanlings and Older Horses but Lower Risk to Young Foals Born to Vaccinated Mares
Diseases of Low Risk to Foals
Adverse Reactions to Vaccines
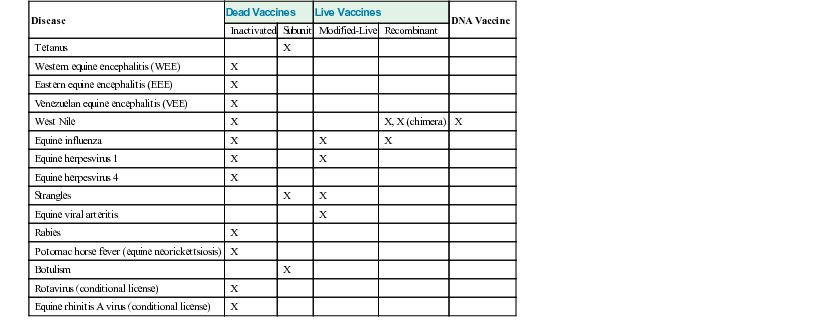
Available Vaccines and the Concept of Core and Non-Core Vaccines
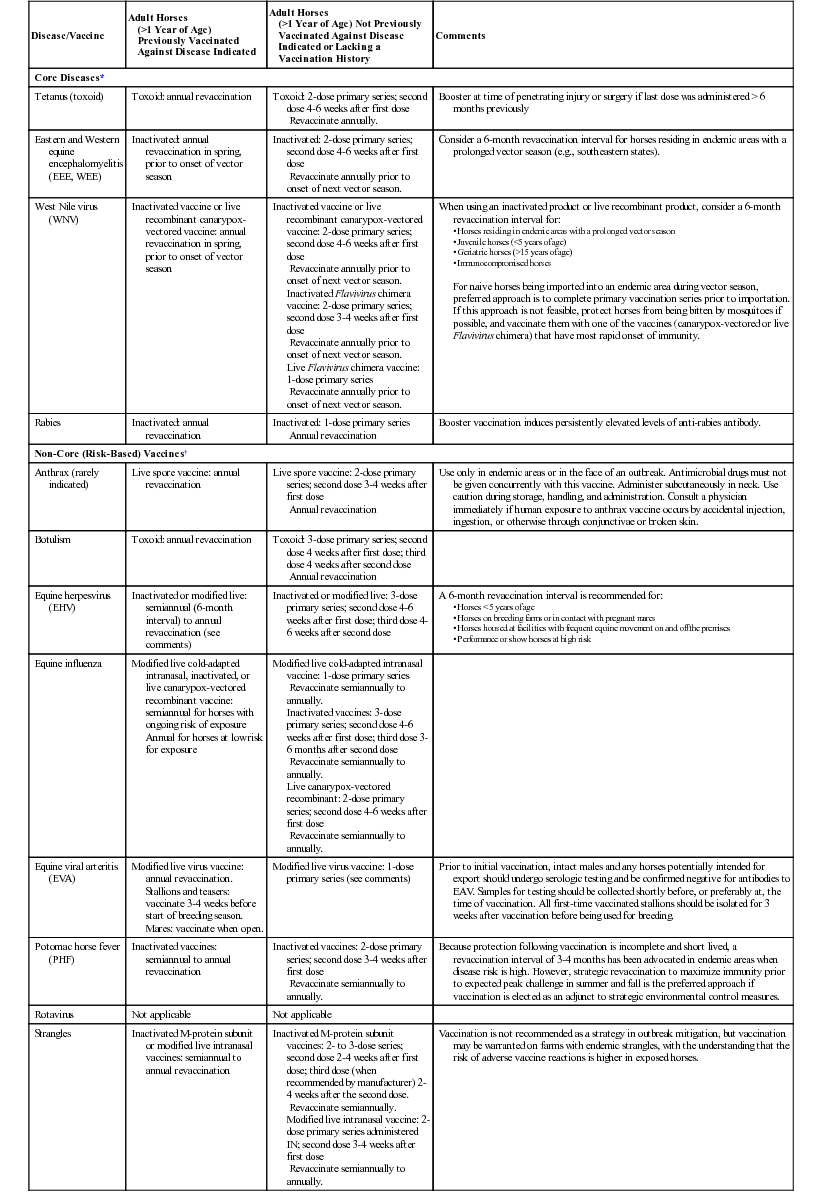
Disease/Vaccine
Adult Horses
(>1 Year of Age) Previously Vaccinated Against Disease Indicated
Adult Horses
(>1 Year of Age) Not Previously Vaccinated Against Disease Indicated or Lacking a Vaccination History
Comments
Core Diseases*
Tetanus (toxoid)
Toxoid: annual revaccination
Toxoid: 2-dose primary series; second dose 4-6 weeks after first dose
Revaccinate annually.
Booster at time of penetrating injury or surgery if last dose was administered > 6 months previously
Eastern and Western equine encephalomyelitis (EEE, WEE)
Inactivated: annual revaccination in spring, prior to onset of vector season
Inactivated: 2-dose primary series; second dose 4-6 weeks after first dose
Revaccinate annually prior to onset of next vector season.
Consider a 6-month revaccination interval for horses residing in endemic areas with a prolonged vector season (e.g., southeastern states).
West Nile virus (WNV)
Inactivated vaccine or live recombinant canarypox-vectored vaccine: annual revaccination in spring, prior to onset of vector season
Inactivated vaccine or live recombinant canarypox-vectored vaccine: 2-dose primary series; second dose 4-6 weeks after first dose
Revaccinate annually prior to onset of next vector season.
Inactivated Flavivirus chimera vaccine: 2-dose primary series; second dose 3-4 weeks after first dose
Revaccinate annually prior to onset of next vector season.
Live Flavivirus chimera vaccine: 1-dose primary series
Revaccinate annually prior to onset of next vector season.
When using an inactivated product or live recombinant product, consider a 6-month revaccination interval for:
For naive horses being imported into an endemic area during vector season, preferred approach is to complete primary vaccination series prior to importation. If this approach is not feasible, protect horses from being bitten by mosquitoes if possible, and vaccinate them with one of the vaccines (canarypox-vectored or live Flavivirus chimera) that have most rapid onset of immunity.
Rabies
Inactivated: annual revaccination
Inactivated: 1-dose primary series
Annual revaccination
Booster vaccination induces persistently elevated levels of anti-rabies antibody.
Non-Core (Risk-Based) Vaccines†
Anthrax (rarely indicated)
Live spore vaccine: annual revaccination
Live spore vaccine: 2-dose primary series; second dose 3-4 weeks after first dose
Annual revaccination
Use only in endemic areas or in the face of an outbreak. Antimicrobial drugs must not be given concurrently with this vaccine. Administer subcutaneously in neck. Use caution during storage, handling, and administration. Consult a physician immediately if human exposure to anthrax vaccine occurs by accidental injection, ingestion, or otherwise through conjunctivae or broken skin.
Botulism
Toxoid: annual revaccination
Toxoid: 3-dose primary series; second dose 4 weeks after first dose; third dose 4 weeks after second dose
Annual revaccination
Equine herpesvirus (EHV)
Inactivated or modified live: semiannual (6-month interval) to annual revaccination (see comments)
Inactivated or modified live: 3-dose primary series; second dose 4-6 weeks after first dose; third dose 4-6 weeks after second dose
A 6-month revaccination interval is recommended for:
Equine influenza
Modified live cold-adapted intranasal, inactivated, or live canarypox-vectored recombinant vaccine: semiannual for horses with ongoing risk of exposure
Annual for horses at low risk for exposure
Modified live cold-adapted intranasal vaccine: 1-dose primary series
Revaccinate semiannually to annually.
Inactivated vaccines: 3-dose primary series; second dose 4-6 weeks after first dose; third dose 3-6 months after second dose
Revaccinate semiannually to annually.
Live canarypox-vectored recombinant: 2-dose primary series; second dose 4-6 weeks after first dose
Revaccinate semiannually to annually.
Equine viral arteritis (EVA)
Modified live virus vaccine: annual revaccination.
Stallions and teasers: vaccinate 3-4 weeks before start of breeding season.
Mares: vaccinate when open.
Modified live virus vaccine: 1-dose primary series (see comments)
Prior to initial vaccination, intact males and any horses potentially intended for export should undergo serologic testing and be confirmed negative for antibodies to EAV. Samples for testing should be collected shortly before, or preferably at, the time of vaccination. All first-time vaccinated stallions should be isolated for 3 weeks after vaccination before being used for breeding.
Potomac horse fever (PHF)
Inactivated vaccines: semiannual to annual revaccination
Inactivated vaccines: 2-dose primary series; second dose 3-4 weeks after first dose
Revaccinate semiannually to annually.
Because protection following vaccination is incomplete and short lived, a revaccination interval of 3-4 months has been advocated in endemic areas when disease risk is high. However, strategic revaccination to maximize immunity prior to expected peak challenge in summer and fall is the preferred approach if vaccination is elected as an adjunct to strategic environmental control measures.
Rotavirus
Not applicable
Not applicable
Strangles
Inactivated M-protein subunit or modified live intranasal vaccines: semiannual to annual revaccination
Inactivated M-protein subunit vaccines: 2- to 3-dose series; second dose 2-4 weeks after first dose; third dose (when recommended by manufacturer) 2-4 weeks after the second dose.
Revaccinate semiannually.
Modified live intranasal vaccine: 2-dose primary series administered IN; second dose 3-4 weeks after first dose
Revaccinate semiannually to annually.
Vaccination is not recommended as a strategy in outbreak mitigation, but vaccination may be warranted on farms with endemic strangles, with the understanding that the risk of adverse vaccine reactions is higher in exposed horses.
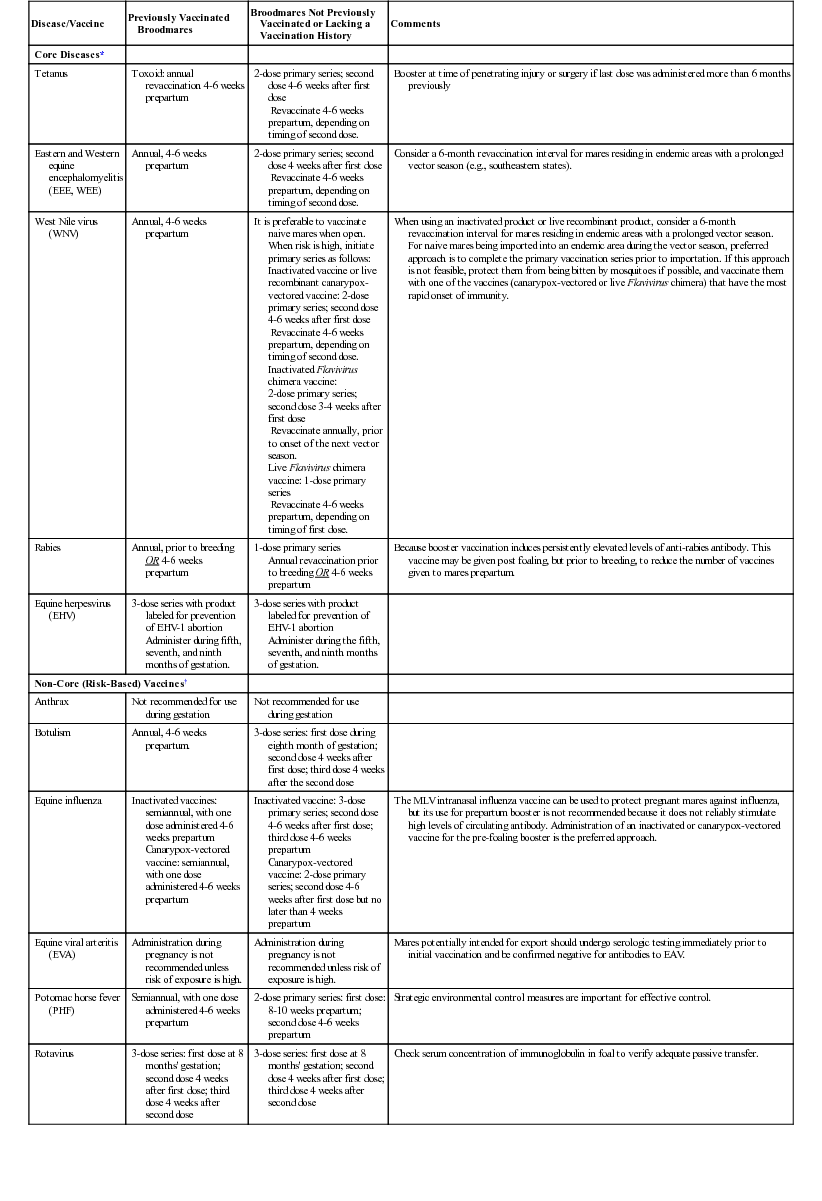
Disease/Vaccine
Previously Vaccinated Broodmares
Broodmares Not Previously Vaccinated or Lacking a Vaccination History
Comments
Core Diseases*
Tetanus
Toxoid: annual revaccination 4-6 weeks prepartum
2-dose primary series; second dose 4-6 weeks after first dose
Revaccinate 4-6 weeks prepartum, depending on timing of second dose.
Booster at time of penetrating injury or surgery if last dose was administered more than 6 months previously
Eastern and Western equine encephalomyelitis (EEE, WEE)
Annual, 4-6 weeks prepartum
2-dose primary series; second dose 4 weeks after first dose
Revaccinate 4-6 weeks prepartum, depending on timing of second dose.
Consider a 6-month revaccination interval for mares residing in endemic areas with a prolonged vector season (e.g., southeastern states).
West Nile virus (WNV)
Annual, 4-6 weeks prepartum
It is preferable to vaccinate naive mares when open. When risk is high, initiate primary series as follows:
Inactivated vaccine or live recombinant canarypox-vectored vaccine: 2-dose primary series; second dose 4-6 weeks after first dose
Revaccinate 4-6 weeks prepartum, depending on timing of second dose.
Inactivated Flavivirus chimera vaccine:
2-dose primary series; second dose 3-4 weeks after first dose
Revaccinate annually, prior to onset of the next vector season.
Live Flavivirus chimera vaccine: 1-dose primary series
Revaccinate 4-6 weeks prepartum, depending on timing of first dose.
When using an inactivated product or live recombinant product, consider a 6-month revaccination interval for mares residing in endemic areas with a prolonged vector season.
For naive mares being imported into an endemic area during the vector season, preferred approach is to complete the primary vaccination series prior to importation. If this approach is not feasible, protect them from being bitten by mosquitoes if possible, and vaccinate them with one of the vaccines (canarypox-vectored or live Flavivirus chimera) that have the most rapid onset of immunity.
Rabies
Annual, prior to breeding OR 4-6 weeks prepartum
1-dose primary series
Annual revaccination prior to breeding OR 4-6 weeks prepartum
Because booster vaccination induces persistently elevated levels of anti-rabies antibody. This vaccine may be given post foaling, but prior to breeding, to reduce the number of vaccines given to mares prepartum.
Equine herpesvirus (EHV)
3-dose series with product labeled for prevention of EHV-1 abortion
Administer during fifth, seventh, and ninth months of gestation.
3-dose series with product labeled for prevention of EHV-1 abortion
Administer during the fifth, seventh, and ninth months of gestation.
Non-Core (Risk-Based) Vaccines†
Anthrax
Not recommended for use during gestation
Not recommended for use during gestation
Botulism
Annual, 4-6 weeks prepartum.
3-dose series: first dose during eighth month of gestation; second dose 4 weeks after first dose; third dose 4 weeks after the second dose
Equine influenza
Inactivated vaccines: semiannual, with one dose administered 4-6 weeks prepartum
Canarypox-vectored vaccine: semiannual, with one dose administered 4-6 weeks prepartum
Inactivated vaccine: 3-dose primary series; second dose 4-6 weeks after first dose; third dose 4-6 weeks prepartum
Canarypox-vectored vaccine: 2-dose primary series; second dose 4-6 weeks after first dose but no later than 4 weeks prepartum
The MLV intranasal influenza vaccine can be used to protect pregnant mares against influenza, but its use for prepartum booster is not recommended because it does not reliably stimulate high levels of circulating antibody. Administration of an inactivated or canarypox-vectored vaccine for the pre-foaling booster is the preferred approach.
Equine viral arteritis (EVA)
Administration during pregnancy is not recommended unless risk of exposure is high.
Administration during pregnancy is not recommended unless risk of exposure is high.
Mares potentially intended for export should undergo serologic testing immediately prior to initial vaccination and be confirmed negative for antibodies to EAV.
Potomac horse fever (PHF)
Semiannual, with one dose administered 4-6 weeks prepartum
2-dose primary series: first dose: 8-10 weeks prepartum; second dose 4-6 weeks prepartum
Strategic environmental control measures are important for effective control.
Rotavirus
3-dose series: first dose at 8 months’ gestation; second dose 4 weeks after first dose; third dose 4 weeks after second dose
3-dose series: first dose at 8 months’ gestation; second dose 4 weeks after first dose; third dose 4 weeks after second dose
Check serum concentration of immunoglobulin in foal to verify adequate passive transfer.
Strangles
Inactivated M-protein subunit vaccines: semiannual, with one dose given 4-6 weeks prepartum
Inactivated M-protein subunit vaccines: 3-dose primary series; second dose 2-4 weeks after the first dose; third dose 4-6 weeks prepartum
The MLV intranasal strangles vaccine can be used to protect pregnant mares, but its use for the prepartum booster is not recommended because it does not reliably stimulate high levels of circulating antibody. Administration of an inactivated M-protein vaccine for the pre-foaling booster is the preferred approach.

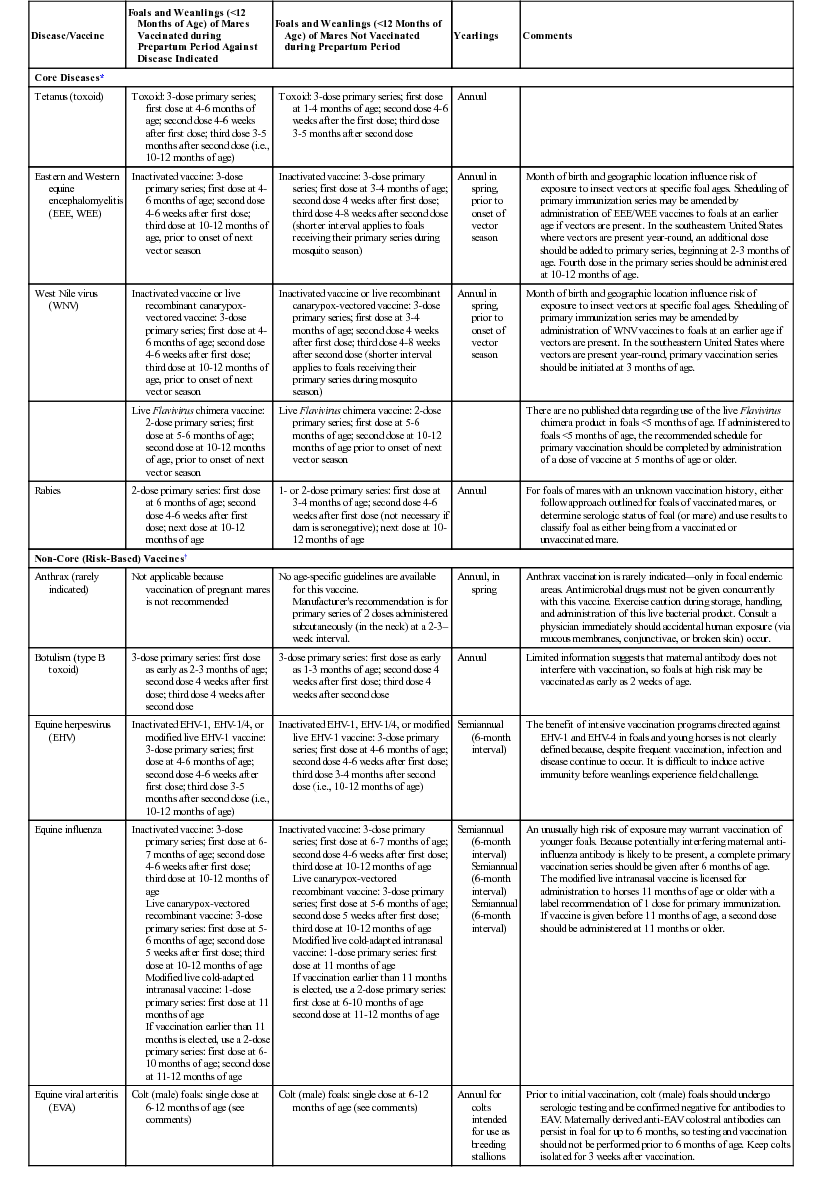
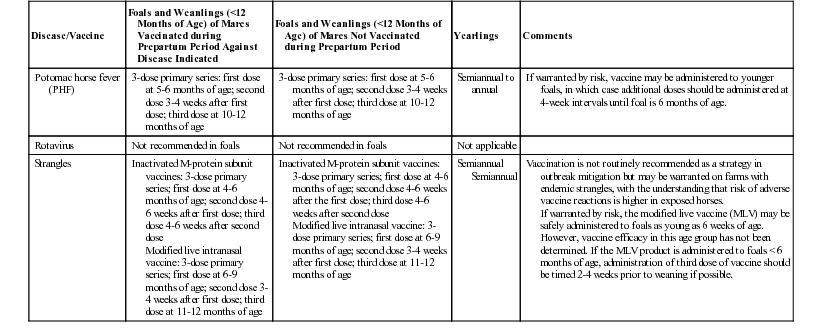
Disease/Vaccine
Foals and Weanlings (<12 Months of Age) of Mares Vaccinated during Prepartum Period Against Disease Indicated
Foals and Weanlings (<12 Months of Age) of Mares Not Vaccinated during Prepartum Period
Yearlings
Comments
Core Diseases*
Tetanus (toxoid)
Toxoid: 3-dose primary series; first dose at 4-6 months of age; second dose 4-6 weeks after first dose; third dose 3-5 months after second dose (i.e., 10-12 months of age)
Toxoid: 3-dose primary series; first dose at 1-4 months of age; second dose 4-6 weeks after the first dose; third dose 3-5 months after second dose
Annual
Eastern and Western equine encephalomyelitis (EEE, WEE)
Inactivated vaccine: 3-dose primary series; first dose at 4-6 months of age; second dose 4-6 weeks after first dose; third dose at 10-12 months of age, prior to onset of next vector season
Inactivated vaccine: 3-dose primary series; first dose at 3-4 months of age; second dose 4 weeks after first dose; third dose 4-8 weeks after second dose (shorter interval applies to foals receiving their primary series during mosquito season)
Annual in spring, prior to onset of vector season
Month of birth and geographic location influence risk of exposure to insect vectors at specific foal ages. Scheduling of primary immunization series may be amended by administration of EEE/WEE vaccines to foals at an earlier age if vectors are present. In the southeastern United States where vectors are present year-round, an additional dose should be added to primary series, beginning at 2-3 months of age. Fourth dose in the primary series should be administered at 10-12 months of age.
West Nile virus (WNV)
Inactivated vaccine or live recombinant canarypox-vectored vaccine: 3-dose primary series; first dose at 4-6 months of age; second dose 4-6 weeks after first dose; third dose at 10-12 months of age, prior to onset of next vector season
Inactivated vaccine or live recombinant canarypox-vectored vaccine: 3-dose primary series; first dose at 3-4 months of age; second dose 4 weeks after first dose; third dose 4-8 weeks after second dose (shorter interval applies to foals receiving their primary series during mosquito season)
Annual in spring, prior to onset of vector season
Month of birth and geographic location influence risk of exposure to insect vectors at specific foal ages. Scheduling of primary immunization series may be amended by administration of WNV vaccines to foals at an earlier age if vectors are present. In the southeastern United States where vectors are present year-round, primary vaccination series should be initiated at 3 months of age.
Live Flavivirus chimera vaccine: 2-dose primary series; first dose at 5-6 months of age; second dose at 10-12 months of age, prior to onset of next vector season
Live Flavivirus chimera vaccine: 2-dose primary series; first dose at 5-6 months of age; second dose at 10-12 months of age prior to onset of next vector season
There are no published data regarding use of the live Flavivirus chimera product in foals <5 months of age. If administered to foals <5 months of age, the recommended schedule for primary vaccination should be completed by administration of a dose of vaccine at 5 months of age or older.
Rabies
2-dose primary series: first dose at 6 months of age; second dose 4-6 weeks after first dose; next dose at 10-12 months of age
1- or 2-dose primary series: first dose at 3-4 months of age; second dose 4-6 weeks after first dose (not necessary if dam is seronegative); next dose at 10-12 months of age
Annual
For foals of mares with an unknown vaccination history, either follow approach outlined for foals of vaccinated mares, or determine serologic status of foal (or mare) and use results to classify foal as either being from a vaccinated or unvaccinated mare.
Non-Core (Risk-Based) Vaccines†
Anthrax (rarely indicated)
Not applicable because vaccination of pregnant mares is not recommended
No age-specific guidelines are available for this vaccine.
Manufacturer’s recommendation is for primary series of 2 doses administered subcutaneously (in the neck) at a 2-3–week interval.
Annual, in spring
Anthrax vaccination is rarely indicated—only in focal endemic areas. Antimicrobial drugs must not be given concurrently with this vaccine. Exercise caution during storage, handling, and administration of this live bacterial product. Consult a physician immediately should accidental human exposure (via mucous membranes, conjunctivae, or broken skin) occur.
Botulism (type B toxoid)
3-dose primary series: first dose as early as 2-3 months of age; second dose 4 weeks after first dose; third dose 4 weeks after second dose
3-dose primary series: first dose as early as 1-3 months of age; second dose 4 weeks after first dose; third dose 4 weeks after second dose
Annual
Limited information suggests that maternal antibody does not interfere with vaccination, so foals at high risk may be vaccinated as early as 2 weeks of age.
Equine herpesvirus (EHV)
Inactivated EHV-1, EHV-1/4, or modified live EHV-1 vaccine: 3-dose primary series; first dose at 4-6 months of age; second dose 4-6 weeks after first dose; third dose 3-5 months after second dose (i.e., 10-12 months of age)
Inactivated EHV-1, EHV-1/4, or modified live EHV-1 vaccine: 3-dose primary series; first dose at 4-6 months of age; second dose 4-6 weeks after first dose; third dose 3-4 months after second dose (i.e., 10-12 months of age)
Semiannual (6-month interval)
The benefit of intensive vaccination programs directed against EHV-1 and EHV-4 in foals and young horses is not clearly defined because, despite frequent vaccination, infection and disease continue to occur. It is difficult to induce active immunity before weanlings experience field challenge.
Equine influenza
Inactivated vaccine: 3-dose primary series; first dose at 6-7 months of age; second dose 4-6 weeks after first dose; third dose at 10-12 months of age
Live canarypox-vectored recombinant vaccine: 3-dose primary series: first dose at 5-6 months of age; second dose 5 weeks after first dose; third dose at 10-12 months of age
Modified live cold-adapted intranasal vaccine: 1-dose primary series: first dose at 11 months of age
If vaccination earlier than 11 months is elected, use a 2-dose primary series: first dose at 6-10 months of age; second dose at 11-12 months of age
Inactivated vaccine: 3-dose primary series; first dose at 6-7 months of age; second dose 4-6 weeks after first dose; third dose at 10-12 months of age
Live canarypox-vectored recombinant vaccine: 3-dose primary series; first dose at 5-6 months of age; second dose 5 weeks after first dose; third dose at 10-12 months of age
Modified live cold-adapted intranasal vaccine: 1-dose primary series: first dose at 11 months of age
If vaccination earlier than 11 months is elected, use a 2-dose primary series: first dose at 6-10 months of age second dose at 11-12 months of age
Semiannual (6-month interval)
Semiannual (6-month interval)
Semiannual (6-month interval)
An unusually high risk of exposure may warrant vaccination of younger foals. Because potentially interfering maternal anti-influenza antibody is likely to be present, a complete primary vaccination series should be given after 6 months of age.
The modified live intranasal vaccine is licensed for administration to horses 11 months of age or older with a label recommendation of 1 dose for primary immunization. If vaccine is given before 11 months of age, a second dose should be administered at 11 months or older.
Equine viral arteritis (EVA)
Colt (male) foals: single dose at 6-12 months of age (see comments)
Colt (male) foals: single dose at 6-12 months of age (see comments)
Annual for colts intended for use as breeding stallions
Prior to initial vaccination, colt (male) foals should undergo serologic testing and be confirmed negative for antibodies to EAV. Maternally derived anti-EAV colostral antibodies can persist in foal for up to 6 months, so testing and vaccination should not be performed prior to 6 months of age. Keep colts isolated for 3 weeks after vaccination.
Potomac horse fever (PHF)
3-dose primary series: first dose at 5-6 months of age; second dose 3-4 weeks after first dose; third dose at 10-12 months of age
3-dose primary series: first dose at 5-6 months of age; second dose 3-4 weeks after first dose; third dose at 10-12 months of age
Semiannual to annual
If warranted by risk, vaccine may be administered to younger foals, in which case additional doses should be administered at 4-week intervals until foal is 6 months of age.
Rotavirus
Not recommended in foals
Not recommended in foals
Not applicable
Strangles
Inactivated M-protein subunit vaccines: 3-dose primary series; first dose at 4-6 months of age; second dose 4-6 weeks after first dose; third dose 4-6 weeks after second dose
Modified live intranasal vaccine: 3-dose primary series; first dose at 6-9 months of age; second dose 3-4 weeks after first dose; third dose at 11-12 months of age
Inactivated M-protein subunit vaccines: 3-dose primary series; first dose at 4-6 months of age; second dose 4-6 weeks after the first dose; third dose 4-6 weeks after second dose
Modified live intranasal vaccine: 3-dose primary series; first dose at 6-9 months of age; second dose 3-4 weeks after first dose; third dose at 11-12 months of age
Semiannual
Semiannual
Vaccination is not routinely recommended as a strategy in outbreak mitigation but may be warranted on farms with endemic strangles, with the understanding that risk of adverse vaccine reactions is higher in exposed horses.
If warranted by risk, the modified live vaccine (MLV) may be safely administered to foals as young as 6 weeks of age. However, vaccine efficacy in this age group has not been determined. If the MLV product is administered to foals < 6 months of age, administration of third dose of vaccine should be timed 2-4 weeks prior to weaning if possible.
Vaccination Recommendations for Specific Diseases
Tetanus
Equine Encephalomyelitis (Sleeping Sickness)
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Use of Biologics in the Prevention of Infectious Diseases
Chapter 48