Darin M. Collins Bears are mammals within the order Carnivora of the divergent family Ursidae and are geographically widespread within North and South Americas, Europe, and Asia. Within Ursidae, eight species of bears exist in three different subfamilies: Ursinae, Tremarctinae, Ailuropodinae (Table 50-1). The recognition of the giant panda (Ailuropoda melanoleuca) as an early divergent from the bear family is now accepted. The subsequent phylogenetic divergence of the spectacled bear (Tremarctos ornatus) to the giant panda, and the grouping of the brown bear (Ursus arctos) and the polar bear (Ursus maritimus) have been accepted. Bears occupy a wide range of ecologic niches from the arctic ice to the tropical rainforests. Wild bears are generally diurnal but may be active during the night (nocturnal) or twilight (crepuscular). Bears have an excellent sense of smell and some are very adept climbers and swimmers. TABLE 50-1 Biologic Information of Bears, Order Carnivora, Family Ursidae * Sun Bears on Borneo (Helarctos malayanus euryspilus) are sufficiently different from those on the Asian mainland and Sumatra, representing the typical form (H. m. malayanus), to warrant subspecific differentiation.38 All bears have been threatened by human encroachment into their habitats and the illegal trade of bears and bear parts, including the Asian bear bile market. One of the greatest threats to bears is human-imposed environmental alterations such as global warming, chemical pollution, and deforestation. Current and future global climate changes are expected to pose greater risks, particularly for the polar bear, because of their reproductive life history traits, including seasonality. Consequently, six of eight species are currently facing the risk of extinction, with the International Union for the Conservation of Nature and Natural Resources (IUCN) classifications ranging from Endangered to Vulnerable. The IUCN lists all bears except the brown bear and the American black bear as vulnerable or endangered, with the brown bear at risk of extirpation in many range countries. The long-term conservation of small, isolated, and increasingly human-impacted bear populations will require innovative, pragmatic, and site-specific approaches. Common characteristics of bears include a large body with stocky legs, a long snout, plantigrade paws with five non-retractile claws, a short tail, and relatively small eyes. Bears generally have brown, black, or white fur of variable length. Many species have white or yellow crescent-shaped markings on the chest thought to be a social signal that may be best seen when bears stand bipedally, as during aggressive encounters or during periods of alert. Bears may become bipedal opportunistically and have been known to carry their young in this way in captivity. The mammae are pectoral. The gastrointestinal (GI) tract is simple. The distal segment of the intestine is marked only by a change in mucosa, and no cecum is present. Kidneys are lobular or reniculate in structure. In place of a renal pelvis, the major calyces drain the minor calyces of each reniculus and join at the proximal end of the ureter. Unique intraoral epipharyngeal pouches occur in bears as bilateral tubular diverticulations of the caudodorsal pharyngeal wall, which are lined with a respiratory-like epithelium and a thick layer of elastic fibers that suggest a role in phonation.61 Bears vocalize using grunts, growls, moans, tongue clicks, blowing, and teeth clacking, and giant panda vocalizations are described more as bleats, barks, and honks. The nursing sound of bear cubs is described as a pulsed “humming” vocalization and thought to be a comfort sound that might stimulate milk release by the lactating female.12 The giant panda possesses a larger radial sesamoid bone compared with that found in other bear species. This specialized bone articulates with the radial carpal and first metacarpal bone and supports the associated pad and muscle attachments. Such an adaptation provides for the gripping mechanism created by the opposing pads of the other four digits during flexion to manipulate objects such as bamboo. Bears generally have elongated crushing premolar (P) and molar (M) teeth with a dental formula: incisors (I) 3/3, canines (C) 1/1, P 4/4, M 3/3. In the giant panda it is: I 3/3, C 1/1, P 4/4, M 2/2. Patterns of craniodental variation in the skulls of bears, as relating to their diet and feeding behavior, reflect dietary adaptations of teeth and the biomechanical properties of the skull, jaw, and related musculature.52 As carnivores, polar bears are distinguished by molar size reduction, flexible mandibles, relatively small carnassial blades, and rounded canines. In contrast, giant pandas, as herbivores, have large molar grinding areas, rigid mandibles, large carnassial blades, and relatively reduced canines. The insectivorous sloth bear has only four upper incisors, with the sizes of post-canine teeth being reduced, and a vaulted hard palate to facilitate feeding by suction. The remaining species, which are omnivores, have a dental morphology intermediate between those of carnivorous and herbivorous species, with mediolaterally compressed, bladed canine teeth and relatively large molar grinding surfaces. Sloth bears and sun bears have soft tissue adaptations related to their insectivorous diet, including long tongues and flexible nares, with reduced hair on the muzzle. Physiologically, bears are remarkable mammals. Wild bears in the Northern Hemisphere during winter months will experience a period of dormancy or torpor, similar to hibernation, a unique state of energy conservation in response to harsh climatic conditions and food scarcity. This dormancy is characterized by inactivity and a lowered metabolic rate, prolonged complete or partial cessation of food and water intake, and absence of defecation or urination, with the possibility of arousal when disturbed. The body temperature of bears does not decrease dramatically during denning. The majority of the energy to support these activities is derived from lipid stores. Pregnant females will also give birth and lactate during this dormancy period. In polar bears, only the pregnant female will undergo this winter denning, and polar bears of either sex may go into torpor during the arctic summer when food is scarce. In captivity, most temperate species lay down fat in the autumn, and winter seasonal food intake is typically decreased with periods of decreased physical activity. This seasonal period of dormancy may be induced in captivity by a controlled decrease of food and hydration and by lowering the ambient temperature.50 Hibernating brown bears evaluated by ultrasonography showed heart rates that were significantly lowered from active to hibernating states, with no difference in diastolic and stroke volume parameters.41 Observed changes in reduced atrial chamber function were proposed as the major adaptation during hibernation; this adaptation allows the myocardium to conserve energy, avoid chamber dilation, and remain healthy during this prolonged period of extremely low heart rates. Urea is hydrolyzed, and nitrogen is processed into amino acids, which enter protein synthesis pathways at an accelerated rate.26 As a result, blood urea does not build up despite the absence of urination. These changes occur independent of gender and reproductive or lactational status. Delayed implantation, or embryonic diapause, is a reproductive strategy used by bears whereby the embryo (blastocyst) does not immediately implant in the uterus and is maintained in a state of suspended dormancy. Obligate diapause is also known as seasonal delayed implantation and is a mechanism that allows mammals to time the birth of their offspring for favorable environmental conditions. Little to no development takes place while the embryo remains within the uterine lumen and unattached to the uterine wall. As a result, the normal gestation period may be extended for a species-specific period. Although much of the molecular regulation involved in activating dormant blastocysts has been characterized, little is still known about the initiation of embryonic diapause, and the conditions which enable a blastocyst to remain dormant. Careful consideration should be given to habitat and off-exhibit housing design to meet the physical, social, behavioral, and psychological needs of the bear species being housed. Bears should be displayed, whenever possible, in exhibits replicating their wild habitat and in numbers sufficient to meet their social and behavioral needs. With the aid of innovative exhibit designs, different feeding strategies, appropriate use of environmental enrichment, and the development of a cooperative husbandry training program, all bear species may be housed in a dynamic and stimulating environment that maximizes their welfare and decreases the potential for the development of stereotypical behaviors. Given the polar bear’s threatened status under the U.S. Marine Mammal Protection Act, additional laws, regulations, and standards of care must be followed.36 Institutions should be familiar with these regulations, have access to the documents containing these regulations, and, where appropriate, fully comply with the standards of care detailed within them. Regulations pertaining to polar bear captive housing are also contained within the U.S. Department of Agriculture (USDA) Animal Welfare Act (AWA) and the Manitoba Polar Bear Protection Act (PBPA).1,44 Institutions seeking to acquire or holding polar bears from the Manitoba region are subject to the regulations stated in the PBPA, with the caveat that all institutions housing polar bears should be aware of and consider the management and housing approaches described. The USDA AWA Animal Welfare Regulations mandate that polar bear primary enclosures housing polar bears consist of a pool of water, a dry resting and social activity area, and a den.1 Minimum specifications for each enclosure feature are stated within the AWA Regulations. For example, the pool of water should have a minimal horizontal dimension of not less than 2.44 meters (m; 8 feet [ft.]) and a surface area of at least 8.93 square meters (96.0 square feet) with a minimum depth of 1.52 m (5 ft.), excepting entry and exit areas. A pool of this size should be adequate for two polar bears. Records must be kept of water quality assessments, with weekly water samples required for coliform counts and daily samples for measuring pH and any chemical additives used to maintain water quality standards. Water quality records must be maintained for 1 year documenting the time of sample collection, with the results being made available for USDA inspection purposes when requested. USDA regulations require that visual health inspections of polar bears be conducted by the institution’s animal care staff at least every 6 months and that a general overall visual health assessment be made and recorded in each animal’s health record. Males and females of all bear species may typically be housed together through the year, with males routinely denied access to females prior to denning for the birth of cubs. Decisions to introduce bears for breeding are based on the temperaments of individual bears, with careful consideration being given to signs of progressive, positive, affiliative interactions observed. Facilities should allow for a range of physical contact situations, beginning with limited physical contact through smaller mesh to widely spaced bars that allow the bears to physically reach through and touch each other. Bear introductions may be aggressive, so full contact introductions should be coordinated with veterinary staff members present or immediately available. Cubbing dens, which are appropriately sized according to species, are confined spaces that are adjacent to larger holding areas in which the female may move around and give birth. Remote monitoring of the den via low-level lighting video cameras and microphones, with remote temperature sensors is recommended. Accommodations for supplemental heat are not typically required inside the den, as heavy bedding may provide for any necessary insulation for the female and the cubs. Diet formulations should address the bear’s nutritional needs, feeding ecology, and individual and natural histories to ensure that species-specific feeding patterns and behaviors are stimulated. In the wild, most bear species are opportunistic omnivores consuming a wide variety of food items depending on seasonal abundance; dietary niche specializations rely on variable seasonal availability of insects, fruits, and plants. The polar bear is mostly carnivorous with occasional consumption of plant matter and the giant panda feeds almost entirely on bamboo, but the remaining six species are classified as omnivorous. The giant panda is a strict herbivore, existing on a diet of bamboo and, unlike most herbivores, does not rely on microbial breakdown of plant material. Bamboo shoots and roots make up most of the diet, and the panda is adapted to consume large quantities of this poor protein source. The sloth bear and the sun bear are adapted to insectivory and feed on termites and other insects in the wild. In captivity, sloth bears and sun bears are typically fed diets similar to those of other bear omnivorous species. In captivity, bears are typically fed mixtures of commercial dog food, carnivore-based diets, and produce. Institutions should determine seasonal diet changes on the basis of regular visual assessment of body condition, body weight trends, and activity of the bears. Obesity may be a common nutritional problem in captive bears. Body condition scoring is a subjective assessment of the relative amount and distribution of body fat to muscle and provides for a common language when monitoring body weight and condition over time. A useful standardized fat index for body scoring polar bears used by field biologists has validated scores against actual body weights.56 Feeding schedules to facilitate shifting or other management needs should be supplemented by irregularly timed feeding opportunities and placing foods in novel locations within the exhibits. Scatter feeding, feeding smaller amounts more often, and using enrichment devices that dispense food or live insects may decrease stereotypical behaviors and provide important physical activity and psychological enrichment. The caloric content for the amounts of enrichment foods such as skins and bones with marrow should be factored into the overall diet. All new diet items should be monitored closely when first provided. The food type, presentation, and order of offering may have implications for dental health in bears when considering how to minimize the organic buildup that may contribute to dental health issues. Food items that are soft should be fed first and items such as bones, fish, or those with hair and skin should be offered last to help remove soft and sticky foods from teeth. Synthetic hard bones, ice blocks, and hard frozen food items may contribute to tooth damage, and their use should be monitored. The proper handling and processing of meat and fish products and meat processed on site must follow all USDA standards.9,10 Because of the presence of fish in many polar bear diets, institutions may supplement polar bear diets with thiamin and vitamin E. This perceived need for supplementation is based on the knowledge that thiamin and vitamin E are broken down in stored, frozen fish. Supplementation of thiamin and vitamin E is based on diets that contain greater than 30% fish. If the diet contains less than 30% fish, then other nonfish food items may be providing the needed nutrients. A safe approach is to supplement the fish portion of the diet at 30 milligrams (mg) thiamin and 100 international units (IU) vitamin E per kilogram of frozen fish. Bears should be considered dangerous and typically require chemical immobilization for safe handling and physical examination. Bear cubs weighing less than 25 kg may be physically restrained with nets or blankets for limited physical examination. Training may facilitate diagnostic examinations, and bears may be trained for nonpainful and minimally painful veterinary procedures such as venipuncture, injections, ultrasonography, inspection of teeth and feet, radiography, and wound cleaning, without the need for anesthesia or physical restraint.6,29,37 Captive bears are not prone to complications during anesthesia because of proven chemical restraint agents, typically known medical histories, and reliable body weight estimates. A careful visual examination should be performed prior to any anesthesia event. Bears are usually isolated individually into separate enclosures without climbing structures prior to chemical immobilization. Bears are monogastrics and may be prone to vomiting upon induction or regurgitation during anesthesia. Recommended food and water fasting times for healthy bears is 12 hours. Providing honey as a distraction technique used for darting or hand injections may facilitate calm inductions. Volume limitations for darting may necessitate the use of potent drug combinations for larger brown bears and polar bears. Many bear species demonstrate seasonal variation in body weight with the deposition of a thick layer of fat over the hindquarters during the winter months, making the shoulder area the preferred location for dart placement. Polar bears may have a thick layer of fat at any time of the year, and the shoulder or neck may be targeted and longer needles used for intramuscular injections. A variety of agents, combinations, and dosages have been used to immobilize captive and wild bears effectively (Table 50-2).2,5,7,34,48 The selection of induction agents is typically based on volumes accommodated by the darting systems available. Considerable variations in the doses and combinations of induction agents used for wild bears, compared with those used in captive bears, tend toward increased doses used in wild bears to compensate for unknown weights and to increase the odds of a quick induction. Most immobilization regimens have consisted of a combination of a dissociative agent and an α2-agonist or benzodiazepine. TABLE 50-2 Chemical Restraint Agents Used for Captive Bears * All immobilization agents are given as intramuscular dosages unless otherwise indicated. † Delivered for oral–transmucosal absorption. IM, Intramuscularly; IV, intravenously; µg/kg, microgram per kilogram; mg/kg, milligram per kilogram. Fort Dodge Animal Health; Fort Dodge, Iowa; Lloyd Incorporated; Shenandoah, Iowa; Pfizer, Inc.; Exton, Pennsylvania; Genentech USA, Inc., San Francisco, CA; Wildlife Pharmaceuticals, Inc.; Fort Collins, Colorado. Lyophilization of ketamine and its reconstitution at a concentration of 200 milligrams per milliliter (mg/mL) permits the immobilization of larger specimens with most darting systems. Ketamine (2.2 milligrams per kilogram [mg/kg], intramuscularly [IM]) may be used to supplement any immobilization regimen if anesthesia time needs to be prolonged. A combination of tiletamine and zolazepam is also used commonly to immobilize bears. This combination has the advantages of being more potent, on a milligram-per-kilogram basis, than ketamine and being available in a powder form. The combination may be reconstituted with variable quantities of diluent, or another immobilization agent, providing effective doses in small volumes. Flumazenil used to reverse the zolazepam effects of this combination may reduce the anesthesia recovery time. Medetomidine and xylazine are commonly used as α2-agonists in combination with a dissociative agent for immobilizing bears to achieve a reliable state of analgesia. Both have advantages as they are commercially available in concentrated forms for dart delivery, are reversible with yohimbine, tolazoline, or atipamezole, and are nonnarcotic drugs. Medetomidine is 10% more potent than xylazine and has a higher α2-agonist receptor affinity that produces sedation and analgesia. Spontaneous muscle contractions and partial arousal may be seen in some animals sedated with medetomidine. Etorphine and carfentanil, ultrapotent opioids, have been used variably to immobilize a number of bear species.23 The advantages of these opioids are the small doses and thus small volumes required and their effects being fully reversible with opioid antagonists. The disadvantages of opioids are profound respiratory depression and concerns about the risk for accidental injection of the personnel involved. Both these disadvantages have resulted in limited use of opioids in bears. Carfentanil, as an induction agent, is mixed with honey or syrup and given slowly to increase mucosal absorption; it has also been administered orally for transmucosal absorption to immobilize black, brown, polar, and spectacled bears.47 An opioid antagonist is delivered via darting, 20 to 25 minutes after carfentanil administration, in conjunction with a combination of a dissociative agent and α2-agonist for sustained immobilization. For prolonged procedures, inhalation anesthetic agents may be used. Young animals may be induced via a face mask. An endotracheal tube typically is placed, with the bear in ventral, dorsal, or lateral recumbency, and is used to maintain anesthesia. Throughout the interval of anesthesia maintenance, measurement of vital signs and full physiologic monitoring, including electrocardiography (ECG), pulse oximetry, evaluating end-tidal carbon dioxide, and tracking blood pressure noninvasively using a cuff around a forelimb, are continuously performed. Body temperature monitoring is vital in the larger species, as hyperthermia may occur. Recovery should be monitored closely, and antagonists should always be used, if available. The use of positive reinforcement training may enable many nonpainful and minimally painful veterinary procedures such as inspection of teeth and feet, radiography, ultrasonography, cleaning of wounds, injections, and blood sampling, without the need for anesthesia or physical restraint. Diagnostic procedures for bears are generally similar to those used for domestic dogs and easily adapted for use in bears. Blood is readily obtained from the jugular vein or the cephalic vein in neonates and adults. The jugular and femoral veins are useful for large-volume blood collection. Catheter placement and stabilization during procedures is most suitable in the cephalic or lateral saphenous vein. The dorsal venous plexus of the forepaws is superficial and of sufficient size to allow for venipuncture, even in the awake bear when training for voluntary venipuncture. Whole body radiography, including head, thoracic, abdominal, and extremity views, is challenging in larger bears because of size, as multiple exposures to cover the entire area are usually necessary and anesthesia time to procedures is increased. Baseline radiographic examinations are recommended during the quarantine period and during examination of otherwise healthy bears. It is challenging to ensure that the x-rays penetrate the abdomen of an adult polar bear or brown bear to obtain high-quality, diagnostic films. Ultrasonography in larger patients is challenging but is easier and routinely performed in the smaller species. Endocavitary probes are commonly used rectally for imaging the reproductive tract. Joints and teeth are also common sites radiographed in bears. Hematology (Table 50-3) and serum biochemistry (Table 50-4) reference values for bears have been determined through compilation of MedARKS records from multiple institutions. In general, no remarkable differences exist between species of bears, and the reference values follow trends seen in other carnivores and domestic dogs. Table 50-3 Reference Values for Hematological Parameters for Selected Members of the Family Ursidae
Ursidae
Biology
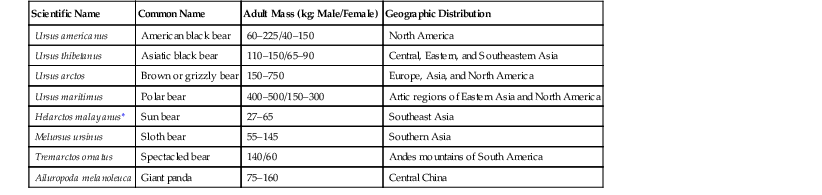
Scientific Name
Common Name
Adult Mass (kg; Male/Female)
Geographic Distribution
Ursus americanus
American black bear
60–225/40–150
North America
Ursus thibetanus
Asiatic black bear
110–150/65–90
Central, Eastern, and Southeastern Asia
Ursus arctos
Brown or grizzly bear
150–750
Europe, Asia, and North America
Ursus maritimus
Polar bear
400–500/150–300
Artic regions of Eastern Asia and North America
Helarctos malayanus*
Sun bear
27–65
Southeast Asia
Melursus ursinus
Sloth bear
55–145
Southern Asia
Tremarctos ornatus
Spectacled bear
140/60
Andes mountains of South America
Ailuropoda melanoleuca
Giant panda
75–160
Central China
Unique Anatomy
Special Physiology
Housing Requirements
Feeding
Restraint and Handling
Chemical Restraint
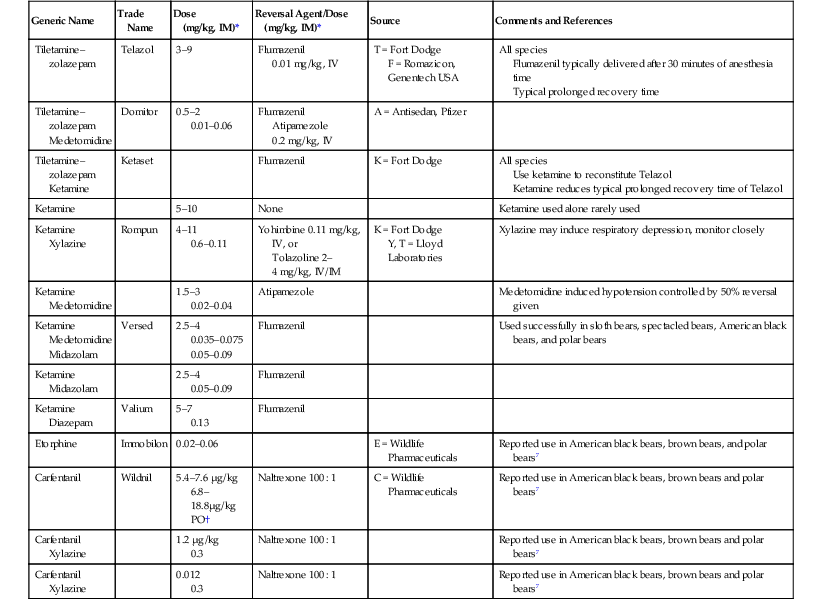
Generic Name
Trade Name
Dose
(mg/kg, IM)*
Reversal Agent/Dose
(mg/kg, IM)*
Source
Comments and References
Tiletamine–zolazepam
Telazol
3–9
Flumazenil
0.01 mg/kg, IV
T = Fort Dodge
F = Romazicon, Genentech USA
All species
Flumazenil typically delivered after 30 minutes of anesthesia time
Typical prolonged recovery time
Tiletamine–zolazepam
Medetomidine
Domitor
0.5–2
0.01–0.06
Flumazenil
Atipamezole
0.2 mg/kg, IV
A = Antisedan, Pfizer
Tiletamine–zolazepam
Ketamine
Ketaset
Flumazenil
K = Fort Dodge
All species
Use ketamine to reconstitute Telazol
Ketamine reduces typical prolonged recovery time of Telazol
Ketamine
5–10
None
Ketamine used alone rarely used
Ketamine
Xylazine
Rompun
4–11
0.6–0.11
Yohimbine 0.11 mg/kg, IV, or
Tolazoline 2–4 mg/kg, IV/IM
K = Fort Dodge
Y, T = Lloyd Laboratories
Xylazine may induce respiratory depression, monitor closely
Ketamine
Medetomidine
1.5–3
0.02–0.04
Atipamezole
Medetomidine induced hypotension controlled by 50% reversal given
Ketamine
Medetomidine
Midazolam
Versed
2.5–4
0.035–0.075
0.05–0.09
Flumazenil
Used successfully in sloth bears, spectacled bears, American black bears, and polar bears
Ketamine
Midazolam
2.5–4
0.05–0.09
Flumazenil
Ketamine
Diazepam
Valium
5–7
0.13
Flumazenil
Etorphine
Immobilon
0.02–0.06
E = Wildlife Pharmaceuticals
Reported use in American black bears, brown bears, and polar bears7
Carfentanil
Wildnil
5.4–7.6 µg/kg
6.8–18.8µg/kg PO†
Naltrexone 100 : 1
C = Wildlife Pharmaceuticals
Reported use in American black bears, brown bears and polar bears7
Carfentanil
Xylazine
1.2 µg/kg
0.3
Naltrexone 100 : 1
Reported use in American black bears, brown bears and polar bears7
Carfentanil
Xylazine
0.012
0.3
Naltrexone 100 : 1
Reported use in American black bears, brown bears and polar bears7
Diagnostics
Parameter
American Black Bear*
Brown Bear*
Polar Bear*
Sun Bear*
Sloth Bear*
Spectacled Bear*
Giant Panda*
Red blood cell count (×106/µL)
7.49
6.33
6.83
5.97
5.85
8.46
6.25
(4.81–9.88)
(4.32–8.35)
(4.53–8.67)
(4.42–7.88)
(4.39–7.38)
(6.50–10.44)
(4.57–7.73)
Hematocrit (%)
45.0
45.8
44.0
41.3
44.9
41.2
35.3
32.3–57.5
(32.6–57.6)
(30.5–55.6)
(30.8–53.9)
(35.5–53.2)
(30.7–52.5)
(26.8–43.5)
Hemoglobin (g/dL)
15.7
16.4
15.6
14.5
15.7
14.6
12.6
(11.2–19.7)
(12.1–21.1)
(11.3–19.3)
(10.5–19.1)
(11.1–19.3)
(11.2–18.1)
(9.5–15.4)
MCV (fL)
59.8
72.6
64.9
70.0
76.5
48.2
58.3
(50.7–67.9)
(62.1–81.9)
(56.4–73.1)
(61.0–81.1)
(68.6–84.6)
(36.0–57.2)
(51.4–64.9)
MCH (pg)
21.0
26.1
23.0
24.6
27.2
17.2
20.3
(17.3–23.8)
(21.3–29.7)
(20.2–25.3)
(21.4–27.2)
(24.3–30.6)
(14.4–19.4)
(18.6–22.0)
MCHC (g/dL)
35.0
36.0
35.3
35.0
34.9
35.4
35.2
(31.0–38.8)
(32.6–39.5)
(31.6–39.0)
(28.0–39.8)
(30.7–40.4)
(29.6–42.2)
(31.0–39.0)
White blood cell count (×103/µL)
8.20
8.04
9.23
10.52
11.46
6.34
7.87
(4.26–15.17)
(4.03–14.18)
(4.77–15.91)
(5.84–17.43)
(5.30–22.14)
(3.59–10.34)
(2.41–12.43)
Neutrophils (×103/µL)
5.32
5.33
6.28
7.45
8.39
4.36
4.54
(2.36–9.83)
(1.96–10.05)
(2.30–11.37)
(3.47–13.74)
(3.62–18.09)
(1.23–7.53)
(0.00–9.75)
Band neutrophils (×103/µL)
0.04
0.04
0.04
0.05
0.06
0.03
0.74
(0.02–0.09)
(0.01–0.08)
(0.02–0.10)
(0.03–0.12)
(0.03–0.12)
(0.01–0.05)
(0.00–0.64)
Lymphocytes (×103/µL)
1.70
1.55
1.51
1.92
1.64
1.27
1.69
(0.40–3.52)
(0.33–4.28)
(0.50–3.39)
(0.44–4.49)
(0.33–3.43)
(0.34–3.12)
(0.15–3.16)
Eosinophils (×103/µL)
0.750 ± 1.035
579
688
549
1027
398
353
(0.012–8.979)
(44–1639)
(82–2230)
(69–1877)
(125–3949)
(51–1220)
(0–913)
Monocytes (×103/mL)
331
434
553
503
494
184
370
(56–943)
(65–1218)
(109–1464)
(91–1384)
(106–1303)
(45–521)
(0–814)
Basophils (×103/µL)
38
—
—
—
—
122
—
(0–105)
—
—
—
—
(0–303)
—
Platelets (×103/µL)
390
352
407
518
487
515
552
(112–687)
(115–595)
(169–649)
(167–871)
(207–799)
(166–840)
(331–787) ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Ursidae
Chapter 50
