Chapter 10
Ultrasonography
Ultrasound has been used in small animal medicine routinely for more than 20 years. It is likely the most important imaging modality other than radiography. Early sonographers of reptile patients adapted this procedure to the special anatomic situations encountered within the wide range of this class.1–4 With continuing advances in ultrasound machines and transducers, the often small size of reptilian or amphibian patients is less of an obstacle. It is possible to perform ultrasound even in small geckos or chameleons, with transducer frequencies of 10, 12, or even 18 MHz. However, several unique considerations still must be kept in mind during ultrasound evaluation of these species.
Equipment
Given the advances in ultrasound technology and equipment over the last several decades, good-quality scanners with high-frequency transducers are available for a reasonable price. A single typically recommended ultrasound equipment setup does not exist because of the wide range of anatomic situations that may be encountered in reptiles. One example of a standard machine is MyLab30Gold Vet (Haslauer-Medizintechnik/Esaote Europe). Depending on the body shape and size, linear, convex, and sector probes may be used (Figure 10-1). For snakes and lizards the linear transducers are generally recommended (Figure 10-2). For chelonians, a sector transducer or convex transducer with a small footprint is advantageous because it fits in the small sonographic windows cranial and caudal to the thoracic limbs and cranial of the pelvic limbs. The frequency of the transducer depends on the size of the animal to be examined. High-frequency transducers have the advantage of superior spatial resolution, yielding excellent detail of superficial structures but have a limited depth of penetration. Lower frequency transducers, although lacking in fine anatomic resolution, have the ability to penetrate deeper into tissue. In small lizards, therefore, high-frequency transducers (10 to 18 MHz) yield the best results. In larger lizards, such as iguanas, 7.5 to 10 MHz linear or convex transducers are recommended. For larger patients such as giant tortoises, frequencies from 2.5 to 5 MHz are recommended.5–14

FIGURE 10-1 Ultrasound probes from left to right: phased array probe 5 to 7.5 MHz; microconvex probe 5 to 8 MHz; linear probe 10 to 18 MHz from Haslauer-Medizintechnik/Esaote Europe.

FIGURE 10-2 Performing ultrasound with a linear probe on a Boa Constrictor in a longitudinal plane; the green light indicates the cranial orientation of the indicator mark.
Special Considerations
It is difficult to accumulate expertise and perform a thorough evaluation when sonographic evaluations are limited to short periods of available time between appointments. Ultrasonography, especially in unfamiliar species, is not a quick procedure. Therefore the office should be comfortable for the sonographer and the client, as well as for the persons handling the patient. A dim but not completely darkened room provides the best environment for viewing the monitor during the examination.
Proficiency in reptile ultrasonography is not easily acquired. Basic technical ultrasound skills needed to use the equipment and accurate interpretation of the findings are necessary. It is very advantageous to have experience in small animal ultrasonography in order to gain familiarity with the scanning technique and operation of the machine. Small animal patients are, in general, easier to evaluate and have a more uniform, familiar anatomy.1–4 When the sonographer has accumulated this basic knowledge, the variable reptile anatomy and the unique sonographic appearances of reptile organs are more easily interpreted. Published literature describing the different reptilian species must be available, and special knowledge should be acquired during seminars, conferences, and laboratories. To improve image interpretation skills, one may also evaluate the organs of postmortem specimens in a water bath (Figure 10-3). This reduces artifacts caused by skin, scales, and other surrounding tissues and allows a more thorough evaluation of the viscera. Any abnormalities detected can be correlated with histopathologic findings (Figure 10-4,) to clarify the etiology of the disease process.

FIGURE 10-3 Water bath for investigating postmortem organs or tumors after surgery; results can be compared with histopathologic findings or can be used for self-training.
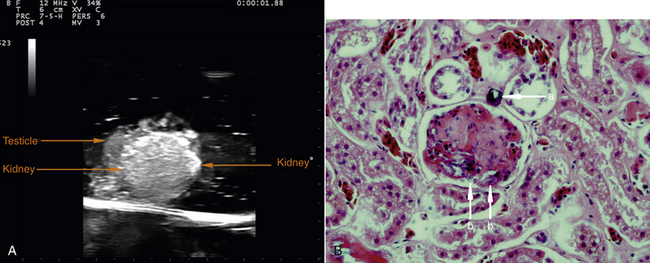
FIGURE 10-4 A, Post-mortem kidney and testicle ultrasound image in water bath; Greek Tortoise (Testudo hermanni). B, Histopathologic diagnosis of changes in kidney of the Greek Tortoise from A. Section of the kidney with calcium microlite (a) and dusty deposition of calcium within the glomerulus (b). Magnification, ×400. (Dr. Anna Kuebber-Heiss, Research Institute of Wildlife Ecology, University of Veterinary Medicine Vienna.)
Patient Preparation
Positioning of the patient must be adapted to what the patient will tolerate while allowing access to the anatomic regions of interest. Typically, dorsal recumbency is the most useful position, but an upright position may also be helpful, especially in weak lizards and in iguanas (Figure 10-5). Large snakes need more personnel to hold them comfortably for this procedure. In aggressive reptiles, sedation should be used to minimize the stress for the animal and the risk to the patient or the technicians.
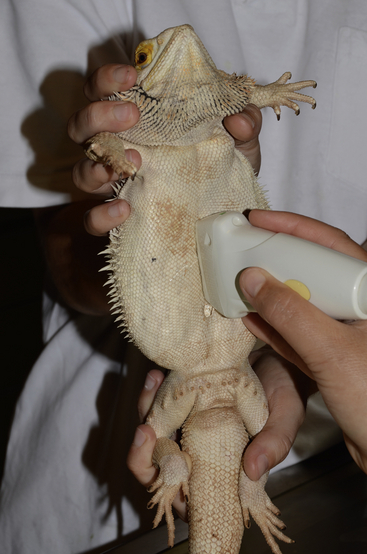
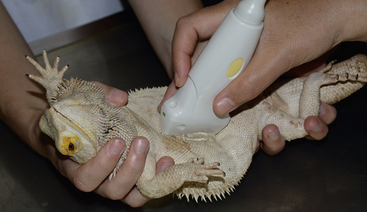
FIGURE 10-5 Upright positioning for weak patients. A Bearded Dragon (Pogona vitticeps) is held while ultrasound is performed.
It is important to develop a routine scanning technique that covers all typically visible organs so that an important sonographic finding is not missed. Whenever possible, each organ should be scanned in two planes: usually transverse and sagittal/longitudinal. Oblique scanning planes are also frequently used to evaluate organs and pathology of variable shape and anatomic location. The probe should always be held in the same fashion such that the sonographer is oriented to the spatial relationship between the tissues being scanned and their appearance on the screen.5–9,13,14 Standard convention dictates that when the patient is in dorsal recumbency, the head is on the left side or facing away from a right-handed sonographer and the transducer is gripped so that the indicator of the probe shows either cranially or to the right side of the patient’s body (Figures 10-6 through 10-9). When this technique is used, the left side of the image on the monitor is cranial in a longitudinal/sagittal view and represents the patient’s right in a transverse view (Figure 10-10).
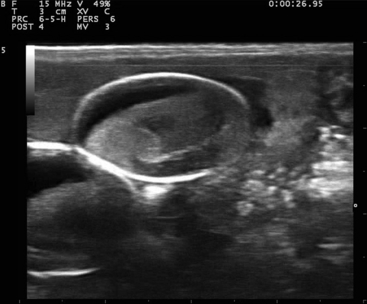
FIGURE 10-7 Sagittal plane image of an egg in the Bearded Dragon (Pogona vitticeps) from Figure 10-6. The hyperechogenic oval structure is the shell, the hypoechoic part is the albumin, and the rest of the content is the developing vitellogenic mass, shortly before being oviposited.

FIGURE 10-8 Positioning of the probe in a transverse imaging plane in a Bearded Dragon (Pogona vitticeps).
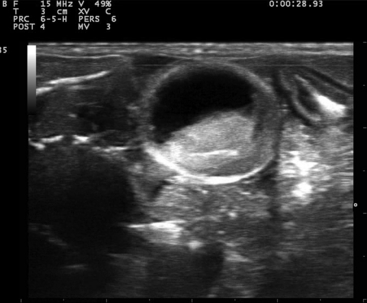
FIGURE 10-9 Transverse plane image of an egg in the Bearded Dragon (Pogona vitticeps) from Figure 10-8.
FIGURE 10-10 In dorsal recumbency, the head of the reptile is on the left side or facing away from a right-handed sonographer, so the indicator of the probe shows either cranially (in this picture) or to the right side of the patient’s body.
The body shape of the snake lends itself to examination from cranial to caudal in longitudinal and transverse scanning planes. In chelonians, there are only three accessible sonographic windows: cranial to the forelimb beside the neck (to investigate the heart and the liver), in the axillary region (for the heart and the liver), and in the inguinal, prefemoral window (to assess the intestine, urogenital tract, and the bladder) (Figure 10-11). In soft-shelled chelonians, it is possible to scan the coelomic viscera through the plastron. This is occasionally also possible in diseased chelonians if they have a poorly mineralized plastron (Figure 10-12).

FIGURE 10-11 Prefemoral window to assess the intestine, urogenital tract, and the bladder in tortoises.
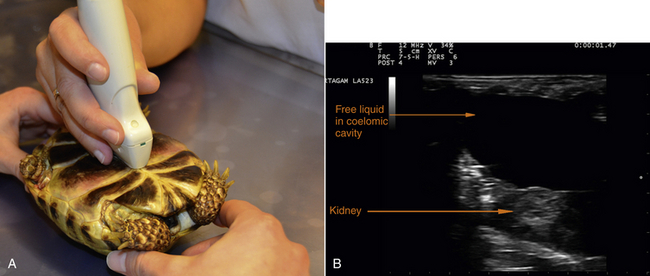
FIGURE 10-12 A, In diseased tortoises, it may be possible to scan the coelomic viscera through the plastron. B, Ultrasound image of the coelomic cavity of a diseased tortoise with renal failure due to secondary nutritional hyperparathyroidism combined with mismanagement of husbandry.
Ample coupling gel must be applied to the skin surface. A gel bottle warmer should be used to prevent cooling of the patient. Better images can be obtained if the skin is moistened with warm water before the coupling gel is applied because contact is improved and trapped air bubbles that attenuate the sound beam are reduced. In chelonians and small patients, a stand-off pad to fill the space between the skin and the transducer surface may be helpful. An examination glove filled with warm water is a useful and substitutes for an inexpensive stand-off pad that can easily conform to the desired shape (Figure 10-13). Coating the standoff pad with coupling gel further enhances the image quality.
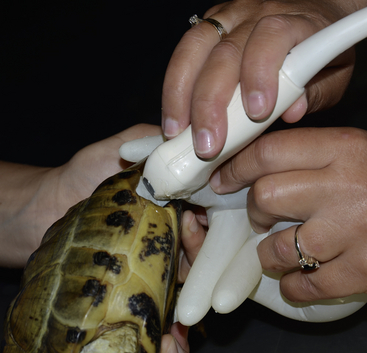
FIGURE 10-13 In tortoises, an examination glove filled with warm water can be used as a stand-off pad in the prefemoral window.
Although ultrasound is an excellent tool to investigate the organs of the coelomic cavity, some circumstances can complicate this procedure. Patients undergoing or soon to be undergoing ecdysis are poor candidates because air between the layers of the skin attenuates the majority of the sound beam, resulting in poor penetration of deeper tissues. Some species with ossified scales (e.g. Tiliqua spp. and Heloderma spp.) are also poor candidates because the ossified scales hinder penetration of the ultrasound beam.
Scanning Pattern
The geometrically simple shape of the snake lends itself to the most straightforward method of sonographic examination. The heart is a useful starting point for establishing appropriate depth and settings on the machine. Blood in the vessels and chambers should be slightly hypoechoic to the cardiac muscle, and the valves should appear as hyperechoic lines. The body must to be scanned in saggital, transverse and dorsal (coronal) views caudally to the level of the cloaca (Figure 10-14).

FIGURE 10-14 The simple shape of the snake lends itself to the most straightforward method of sonographic examination from cranial to caudal. All three planes should be scanned: sagittal, transverse, and dorsal.
Chelonians, except for soft-shelled species, are a little more challenging because they only have three useful acoustic windows to the coelomic cavity.12
The individual coelomic viscera are investigated by measuring the size, shape, and margination of each organ. Changes in echogenicity, either focal or diffuse, can be visualized and interpreted. The gastrointestinal tract is investigated as in small animals for motility, mural thickness, patency, and foreign content. With high-frequency transducers, intestinal wall layering can be appreciated (Figure 10-15).
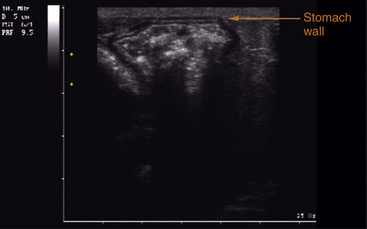
FIGURE 10-15 Ultrasound image of the different layers of the wall of the stomach of a Savannah Monitor (Varanus exanthematicus). The first layer is the small hyperechoic line of the serosa, followed by the low echogenic muscularis layer; next is the higher echogenic submucosa, then the less echogenic mucosa, and the last layer is the highly echogenic mucosal surface, beneath which is seen the content of the stomach as an heterogeneous mass with some parts showing distal attenuation of the ultrasound beam (gas).
The gonads are to be evaluated for size and shape in order to interpret the reproductive stage in both sexes. The kidneys are monitored for size and changes in echogenicity. The urinary bladder, when present, should be investigated for foreign material (e.g., calculi or ectopic eggs). Free coelomic fluid can be detected and assessed for echogenicity, amount, and cellular or solid content.
Indications
Coelomic enlargements of unclear origin (e.g., tumors and ascites), along with reproductive monitoring, are the most common indications for ultrasonography. Any kind of enlargement on the animal’s body can be scanned and interpreted to better characterize the nature of the swelling. Ultrasound-guided aspirates and biopsies may also be performed, but the need for these procedures should be evaluated in conjunction with possible risks such as organ perforation and hemorrhaging.15,16 Reptile echocardiography is a relatively young field, but with improving equipment and continuing publications, the practical clinical utility of this will continue to increase.
The Heart
Reptilian hearts, except those of the crocodilians, have three chambers: two atria and one (normally) thick-walled ventricle, which should not be mistaken for hypertrophic cardiomyopathy. The ventricle is divided by muscular septae in three subchambers: the cavum venosum, cavum pulmonale, and cavum arteriosum. The blood flows from the sinus venosum where the venae cavae join before emptying into the right atrium. From the right atrium, the deoxygenated blood flows to the cavum venosum and further on to the cavum pulmonale, which is the most ventral portion of the chamber. From there, blood passes via the pulmonary arteries to the lung. Oxygenated blood flows from the venae pulmonales to the left atrium and from there to the cavum arteriosum. During systole, the blood is circulated through the still partially contracted cavum venosum in the main circulation arteries.17–20 All reptiles also have paired aortic arches, which are united to the aorta dorsalis. The atrioventricular valves are aligned such that they partially occlude the interventricular canal during atrial systole. During ventricular systole, they should prevent regurgitation of blood from the ventricle to the atria.
The most useful plane for investigating the reptilian heart is the sagittal plane (Figure 10-16, A, B). The second plane is the short-axis view, which is obtained by holding the transducer in a tranverse position, scanning the heart and the vessels from caudal to cranial (Figure 10-16, C, D). If the heart is cranially positioned, as it is in many lizards, it may be easier to view the heart from the left or right axillary region. For tortoises, the cervicobrachial window is applicable (Figure 10-17).
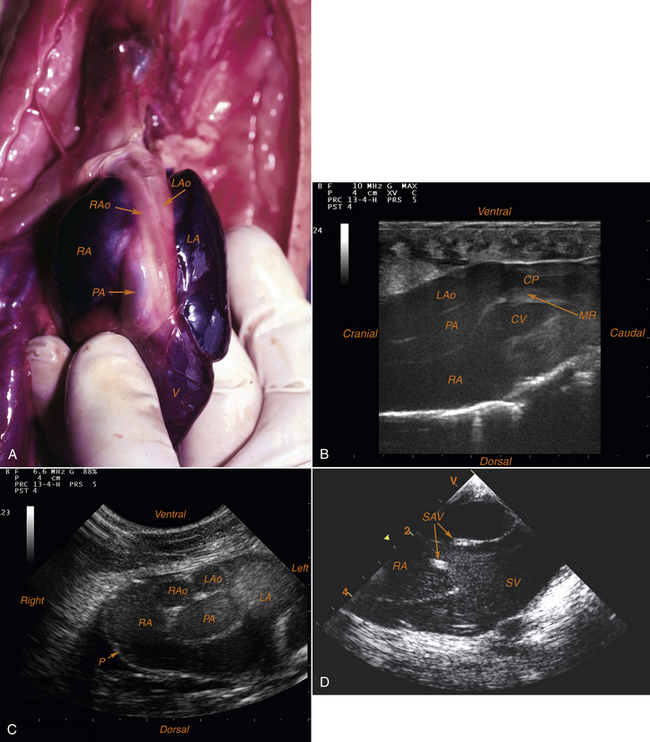
FIGURE 10-16 A, Ventral aspect of the heart in a Burmese python (Python molurus bivittatus) after resection of the pericardial sac. B, Echocardiographic examination in a Red-tailed Boa (Boa constrictor imperator). Long-axis view, right atrioventricular section. C, Echocardiographic examination in a Ball Python (Python regius). Short-axis view, transarterial section “Mickey Mouse Head” section (Photographs A-C courtesy L. Schilliger.) D, Echocardiographic examination in a Burmese Python (Python molurus bivittatus). Short-axis view, transatrial section (thickness because of suspected endocarditis). (Photograph courtesy V. Cherboul.) CP, Cavum pulmonale; CV, cavum venosum; LA, left atrium; LAo, left aortic arch (left aorta); MR, muscular ridge; P, pericardium; PA, pulmonary artery; RA, right atrium; RAo, right aortic arch (right aorta); SAV, sinoatrial valves; SV, sinus venosus; V, ventricle.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree