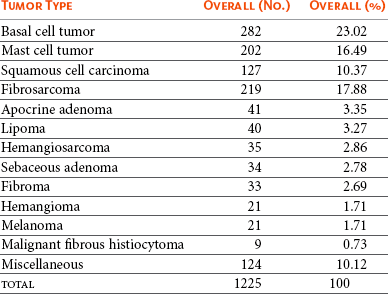
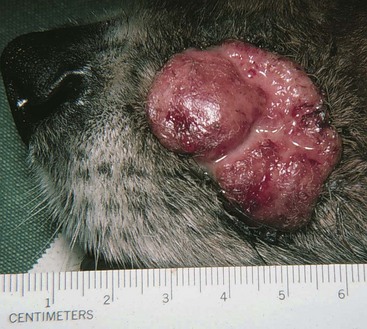
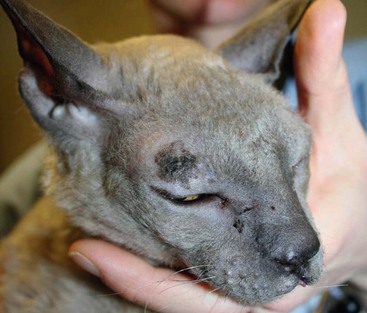
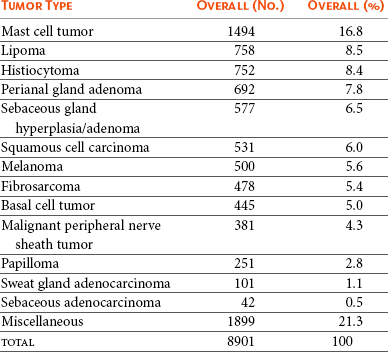
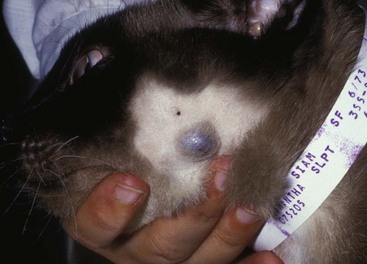
18 The overall incidence of tumors of the skin and subcutaneous tissues of dogs and cats is difficult to determine due to inconsistency of reporting, particularly with tumors of the subcutaneous tissues. If one considers only those tumors determined to be of “skin” origin, the percentage of biopsy specimens in this category has been reported to be 25.5% to 43%.1–6 Of these skin tumors, between 20% and 40% are malignant.2,3 In a survey of neoplasms in the California counties of Alameda and Contra Costa, performed from 1963 until 1966, the estimated incidence of skin and connective tissue tumors was 150.4/100,000 dogs.4 If one considers only nonmelanoma skin tumors, the incidence is calculated at 90.4/100,000 dogs. This same study places the incidence of skin and connective tissue tumors in cats at 51.7/100,000 cats.4 In one report, skin tumors were 9.6% of all feline biopsy or necropsy accessions; however, skin tumors account for 29.6% of all cancer.5 Other studies report a similar percentage of tumors arising from the skin, from 19.3%6 to 21%.7 Disregarding basal cell tumors (BCTs), the percentage of skin tumors that are malignant is much higher in cats than dogs, with studies reporting from 69.7%6 to 82%.5 The relative prevalence of the most common tumor types in dogs and cats can be determined from multiple studies.1,2,7–14 In dogs, the numbers are based on a fairly large number of surveys of skin tumor types from across the globe, totaling almost 9000 skin tumor submissions to various pathology services. The data for the prevalence of the most common tumors of dogs are presented in Table 18-1. The overall prevalence of lipomas and sebaceous adenomas is likely higher than reported as a result of the bias present in samples submitted for histopathologic evaluation. The data on the prevalence of feline tumors are compiled from four studies with a total of 1225 feline skin tumors and are presented in Table 18-2.5,6,9,15 In cats, the top four tumor types of the skin and subcutaneous tissues are consistently BCTs, mast cell tumors, squamous cell carcinoma (SCC), and fibrosarcoma. These four tumor types make up on average approximately 70% of all feline skin tumors. Table 18-1 *Overall incidence of the most common canine skin tumors as determined from the collation of 10 worldwide studies. Ionizing radiation and thermal injury have been reported to increase the risk of skin cancer in many species. A recent epidemiologic study on the incidence of cancer in human burn victims demonstrated no increased risk of the development of skin cancer when compared to the general population, however.16 Ultraviolet (UV) radiation has long been known to cause neoplastic transformation in the skin and is a major contributor to rising rates of skin cancer of all subtypes in humans.17 Evidence for the role of UV irradiation in the development of skin tumors in cats and dogs is primarily epidemiologic in nature18–20 and supported by case reports on dogs diagnosed with a spectrum of sunlight-induced lesions.21,22 The association between SCC development and solar exposure of skin in light-colored cats has been established epidemiologically. Dorn and colleagues calculated that a white cat in California had a 13.4-fold increased risk of developing SCC, and 143 of the 149 cases of nonoral SCC in this study occurred on the head or neck.18 Similar results were found in a case series of nasal planum or pinnal SCC in cats: of the 61 cats, 58 were white or partially white in color and all but three spent time outdoors.23 The ability to induce neoplastic transformation in mucosal infections of papillomaviruses of humans is well established. Papillomaviruses are only able to replicate in terminally differentiated cells; therefore infection of the keratinocyte can stimulate increased proliferation and terminal differentiation.24 Neoplastic transformation arises from the viral effects on cell proliferation, integration into the genome, and interaction of papilloma viral proteins with cellular proteins, particularly the destabilization of p53 by viral protein E6 and the inhibition of pRB by viral protein E7.25 This disruption in p53 can result in increased levels of p16 protein, which is detectable with immunohistochemistry (IHC).26 The association between papillomavirus infection and cutaneous SCC in humans is primarily epidemiologic in nature—organ transplant recipients and immunosuppressed individuals have an increased rate of cutaneous papillomavirus infection and increased risk of SCC development.27,28 The association between canine oral papillomavirus and the development of oral papillomas has been studied since the 1950s.29 The association between viral infection and the development of SCC has evolved from a combination of evidence, including the detection of canine papillomavirus in oral and cutaneous SCCs and the induction of cutaneous SCC in 10 beagles—out of 4500 vaccinated—with a live oral papillomavirus vaccine.30–32 Canine oral papillomavirus has also been detected in multiple cases of cutaneous SCC.31 A novel papillomavirus with malignant potential was cloned from a dog with footpad papillomas.33 Dogs persistently infected with this novel virus developed invasive and metastatic SCC.34 Several additional novel canine papillomaviruses were detected in SCC from a variety of locations, including four cutaneous tumors.35 Case reports support the correlation between papillomavirus infection and the development of invasive SCC, including lesions of mixed histology.32,36,37 Similar to the epidemiologic studies in immunosuppressed humans, a case report of a patient on ongoing chemotherapy developing cutaneous papillomavirus infection and multiple papillomas supports the necessity of immunosuppression in allowing persistent infection of papillomavirus.38 Susceptibility to infection may also depend on the breed.39,40 Recently, a dog with multiple viral plaques developed more than 20 invasive cutaneous SCC over a 3-year period, with no evidence of underlying immunosuppression.41 A novel papillomavirus was sequenced from these lesions. In cats, papillomaviruses are associated with viral plaques and feline fibropapillomas (sometimes referred to as feline sarcoids).42,43 Papillomavirus can be detected with IHC in the majority of feline viral plaques, and as these plaques progress to SCC, the ability to detect papillomavirus antigens decreases.44 However, when polymerase chain reaction (PCR) is used to amplify papillomavirus DNA, up to 76% of “UV-protected” SCCs are positive in comparison with 42% of SCCs in regions exposed to UV irradiation.26 In humans, it has been suggested that UV exposure and papillomavirus infection may act as co-factors in the development of SCC. A large retrospective study tested for the presence of papillomavirus in 126 SCCs, SCCs in situ, or Bowen’s in situ carcinoma (BISC) in 84 cats.45 These investigators found no correlation between likelihood of papillomavirus infection and UV exposure. A novel feline papillomavirus has been sequenced from three feline BISC lesions.46 A total of 25% of the 73 cutaneous lesions were positive for papillomavirus by PCR. Human papillomaviruses 5, 21, and 38 were identified in approximately half of the virus-positive cats. A second study evaluated the levels of p16 in 60 cats; tissues tested were comprised of 14 viral plaques, 14 BISC, 18 invasive SCCs, and 14 trichoblastomas (controls).26 Eleven of the invasive SCCs were solar-induced, and seven were classified as non–solar-induced tumors. P16 protein levels were compared to the trichoblastomas and solar-induced SCC and were found to be elevated in all viral plaques, Bowenoid tumors, and non–solar invasive SCC, which is consistent with the presence of papillomavirus infection. Taken together, these data support the possibility of feline papillomaviruses’ role in the development of SCC in cats. Immunosuppressed humans have a greatly increased risk of skin cancer, whereas organ transplant recipients have up to 100-fold increased risk for development of SCC.47 Although this may reflect susceptibility to persistent papillomavirus infection in some instances, it is also thought to reflect loss of normal immune surveillance, with resulting lack of an immune response against early neoplasia. A case report of the development of multiple cutaneous hamartomas and SCC in situ in a dog receiving long-term immunosuppressive therapy with prednisone and cyclosporine also demonstrated positive staining for papillomavirus antigens.48 Interestingly, the lesions persisted and new lesions developed, even after discontinuation of the drug therapy. The successful use of immune stimulants for early cancer lesions such as imiquimod for carcinoma in situ supports the role of the immune system in controlling skin cancer. The ability of the immune system of dogs to cause regression of histiocytomas and papillomas also illustrates the role of immune surveillance in veterinary patients.49 Cancer is a genetic disease (see Chapter 1, Section A). The accumulation of multiple alterations in critical genes is usually necessary for full neoplastic transformation. An understanding of the genetic abnormalities, including both genetic and epigenetic modifications in a particular cancer type, allows for the formulation of therapeutics to circumvent these critical mutations and their pathways. The accumulation of such genetic data is only beginning in veterinary oncology. The discovery of activating mutations in the stem cell factor receptor, c-KIT, in canine mast cell tumors and the subsequent successful targeting of this mutation with tyrosine kinase inhibitors (TKIs) is the first step in applying this approach in veterinary oncology. Current understanding of mutations present in the most common forms of skin cancer, and their role in either tumorigenesis or prognosis/response to treatment is presented according to tumor type. In humans, basal cell carcinomas (BCCs) are thought to arise from critical mutations in the hedgehog signaling pathway.50 Very little genetic evaluation of BCTs has been performed in veterinary medicine. However, one study demonstrated a reciprocal translocation in a canine BCT: t(10;35).51 In the dog, chromosome 10 contains the gene GLI1, which is the effector transcription factor of the hedgehog signaling cascade. Two aberrant karyotypes were found in feline BCTs: trisomy E3 and monosomy E3, although the significance of these findings are unknown.52,53 IHC for p53 in five feline BCTs were negative.54 Likewise, IHC evaluation for the presence of the apoptotic regulatory proteins Bcl-2 and Bax showed 23 of 24 tumors expressed Bcl-2, only 7 of the BCTs expressed Bax.55 Interestingly, Bcl-2 staining is considered specific for human BCC.56 The genetic abnormalities responsible for the development of cutaneous SCCs are incompletely understood in human oncology. One common finding is mutations in p53. A proposed pathway of sequentially necessary mutations was proposed by Burnworth et al.57 In addition, several genetic changes in cutaneous SCCs have been suggested to be of prognostic value in humans with this disease. There are a number of studies evaluating p53 in canine and feline cutaneous SCCs. When p53 is present in the wild-type form, its short half-life prevents detection of the protein with IHC. The presence of a detectable form of the p53 protein correlates with mutations within the coding sequence. A series of three IHC studies of p53 in canine cutaneous SCCs revealed that 29.5%, or 19 of 65 tumors studied, were positive for p53 overexpression.31,54,58 Interestingly, two of six cutaneous papillomas were also positive for p53 expression by IHC.31 Detectable expression is even more prevalent in feline cutaneous SCCs, with 19 of 40 cutaneous SCCs (47.5%) positive for p53 expression in three studies.53,54,59 In addition, feline actinic keratosis was also found to be highly positive for p53 expression (11 of 14 cases), whereas BISC lesions were less commonly positive (4 of 22 cases).60 These studies have raised questions as to the relative roles of papillomavirus infection and UV irradiation in the development of different subtypes of feline SCCs because both mechanisms of tumorigenesis can result in increased identification of p53. Several studies evaluated changes in selected protein expression with IHC. For example, immunohistochemical staining of p27, a protein thought important in maintaining cells in G0, showed SCCs to have much lower levels than benign cutaneous neoplasms.61 β-Catenin is a protein responsible for normal skin homeostasis. When dysregulated, β-catenin can be oncogenic. A study of β-catenin expression in normal skin and benign and malignant tumors demonstrated nuclear presence of this protein, representing pathway activation, in 100% of the trichoepithelioma and pilomatricoma; no nuclear expression was found in any of the other malignant tumors or normal skin.62 This finding led the authors to suggest a role for aberrantly activated β-catenin in the formation of tumors of the hair follicle. Evaluation for the presence of cyclin D1 and cyclin A, which are proteins important in the regulation of the cell cycle, demonstrated that cyclin A was present in 90% of feline SCCs and 44% of canine SCCs. Cyclin D1 was rarely expressed in skin tumors of any type.54 Staining for these proteins in normal skin and benign tumors showed rare or weak staining. The investigators suggested a role for cyclin A in the regulation of proliferation and neoplastic transformation in cutaneous SCCs. The syndrome of renal cystadenocarcinoma and nodular dermatofibrosis (RCND) deserves mention because this disease often presents to the veterinarian as the result of the manifestation of multiple firm cutaneous nodules. Although first described as a genetic disease in the mid-1980s, it was not until 2003 that the causative mutation in the Birt-Hogg-Dubé (BHD) gene was described.63,64 The BHD gene codes for the tumor suppressor protein folliculin, and mutations in this gene are thought to lead to loss of function.65 Although mostly seen in German shepherd dogs, this syndrome has been reported in Alsatians as well.66 Identification of the driver mutations underlying neoplastic transformation in the skin will be key to optimizing the use of drugs that target these pathways and avoiding unwanted cutaneous side effects. The use of pathway-specific drugs may have unanticipated effects in the skin. Sorafenib, a TKI of Raf and vascular endothelial growth factor/platelet-derived growth factor receptor (VEGF/PDGFR), has been associated with the rapid development of actinic keratosis and invasive SCCs in humans.67,68 As better understanding of these pathways and their role in normal skin homeostasis develops, it may be possible to prevent these types of unintended consequences. Skin tumors arise from the epidermis and associated structures. For ease of use, these tumors are divided based on their differentiation into specific subelements of the skin.69 Some histologies are divided into benign and malignant forms, based on known clinical and histologic predictors of behavior. In other tumor types, such clear-cut division may not be possible. The World Health Organization (WHO) classification system of tumor-node-metastasis can be applied to skin tumors in the clinical setting.70 Box 18-1 describes the application of this staging system to skin tumors. Location is also prognostic for particular tumor types; for example, melanomas of the oral cavity are often malignant, which differs from the expected behavior of a melanoma of haired skin, so this information should be included in the clinical description of the tumor at presentation. Staging involves determination of the extent of disease locally, regionally, and distantly. Assessment of primary tumor size with measurement of the longest diameter is the first step in the staging process. For large, infiltrative, or fixed masses, local assessment may require advanced imaging such as a computed tomography (CT) scan or magnetic resonance imaging (MRI) to accurately determine tumor size and extent. One study demonstrated that the use of advanced local imaging techniques increased the stage of the primary tumor in 69% of patients.71 Regional staging involves the assessment of the draining lymph node(s). Determination of the draining node can be difficult for some locations, so evaluation of all nodes in the region may be necessary. Metastatic lymph nodes may be a normal size and consistency on palpation. Conversely, large, firm nodes may be reactive in response to an infection or inflammatory process. Aspiration and cytologic examination by an experienced clinical pathologist is critical for the assessment of regional lymph nodes for evidence of metastasis. Despite careful evaluation of a cytology sample, it is possible to miss metastatic disease. In cases of questionable cytologic results, histopathologic evaluation of the lymph node is recommended. Given their external location, the primary treatment option to achieve local control of most skin and subcutaneous tumors is surgery. For benign masses, marginal excision may be adequate to achieve long-term control. For malignant tumors, adequate surgical excision requires a margin of normal tissue around the neoplasm. In order for a pathologist to determine if excision is complete, all surgical margins must be identified, with surgical ink or sutures, for correct reporting of margins to occur (see Chapter 9).72 The surgeon or clinician must properly prepare the sample to allow the pathologist to report all critical information, including margin evaluation. A more complete discussion of this topic is reviewed by Kamstock et al.72 The most common cutaneous and subcutaneous tumors, melanomas, mast cell tumors, and soft tissue sarcomas, are discussed in Chapters 19, 20, and 21, respectively. The remainder of this chapter will cover the additional skin tumors, focusing on those with malignant behavior. Tumors of the Primitive Follicular Epithelium The term basal cell tumor (BCT) was used for many years to include BCCs, basal cell epithelioma, trichoblastoma, and solid-cystic ductular sweat gland adenomas and adenocarcinomas. As pathologists’ abilities to differentiate these tumors on the basis of keratin and other membrane markers has progressed, trichoblastomas and solid-cystic ductular sweat gland tumors are no longer considered “basal cell” tumors. As a consequence, older studies that reported high rates of BCT, particularly in cats, may not be reflective of diagnostic patterns today.73 These related tumor types are believed to arise from stem cells in the outer follicular root sheath, displaying variable differentiation, although the origin for all tumors in this category cannot be absolutely identified. Basosquamous cell carcinoma is a tumor with characteristics of both BCCs and SCCs. Immunohistochemically, basosquamous cell carcinomas are more closely related to BCCs and will be discussed in this section. Trichoepitheliomas are a more differentiated form of the trichoblastoma. The most appropriate nomenclature and classification schema for this group of tumors remain controversial. Tumor types will be presented as categorized in the Armed Forces Institute of Pathology publication on the histologic classification of skin tumors.69 The true incidence of BCCs in both dogs and cats is unknown. The different tumors previously categorized as BCTs are difficult to distinguish histologically and cytologically—both the category of tumor and differentiating benign lesions from malignant. On cytology, BCTs can contain inflammatory cells, squamous cells, sebaceous epithelial cells, melanin, and melanophages, and cells can express the criteria of malignancy. Well-differentiated fibroblasts, reactive fibroblasts, and mast cells may also be present on cytologic examination.74 The inability to distinguish the subtypes on cytology has led to the suggestion these tumors be called cutaneous basilar epithelial neoplasms when evaluated by cytology alone.75 Based on histopathologic evaluation, tumors sometimes can be grossly differentiated based on growth pattern.76 The epithelial membrane glycoprotein BerEP4 is highly specific for BCCs versus SCCs or cystic-solid ductular tumors in the diagnosis of BCC in humans.56 Cytokeratin 8 (CAM 5.2) is used with human tumors to identify tumors with sweat gland epithelial differentiation and has been used to differentiate a BCC from a solid-cystic ductular tumor in a dog.75 However, validation of these immunohistochemical markers in a larger veterinary population remains to be performed. BCCs are thought to be rare in dogs. In two studies reporting BCCs or basal cell epithelioma (the benign variant), the incidence ranged from 5.5% to 8.4% of all skin tumors; however, it is unclear if trichoblastomas were included in these populations.10,11 Breeds reported to be at increased risk for BCCs included cocker spaniels and poodles in one study; however, another study reported no breed predispositions. Clinically, these tumors present as plaques or nodules, often darkly pigmented. The overlying skin may be alopecic and intact or ulcerated. In dogs, the median age of patients was reported to be 9 years; however, dogs of all ages may be affected10,11 (Figure 18-1). There are three recognized histologic subtypes: solid, keratinizing, and clear cell.76 In dogs, BCCs are considered a low-grade malignancy. Although there are reports of local recurrence of this tumor after surgical excision, no reports of metastasis in the dog could be found. The application of morphometric analysis of cell nuclei has been reported to be useful in differentiating BCCs potentially able to recur from those that are not likely to recur.77 In cats, BCCs are now thought to be rare, with many of the tumors previously diagnosed as BCTs actually falling into the categories of solid-cystic apocrine ductular adenoma (approximately 60%) and trichoblastoma (approximately 40%).78 However, given the preponderance of the literature referring to “basal cell tumors” in cats, this group of tumors will be discussed here, realizing the population is actually mixed. These tumors comprise some of the most common solid tumors in cats, second only to mammary tumors in one large study.79 They represent 10% to 26% of feline skin tumors.5,9,79,80 These are tumors of middle-aged cats, with a reported mean age of 9.6 to 10.8 years.5,6,80 Although one study reported a predisposition to BCT in Siamese cats, other studies did not find breed differences. BCCs can appear anywhere on the body but may have a predilection for the head and neck. BCC can appear pigmented and resemble a melanoma (Figure 18-2). • Figure 18-2 Firm, circumscribed, pigmented basal cell tumor (likely an apocrine ductal adenoma or a trichoblastoma) on the face of cat. Clinically, the majority of tumors classified as BCT appear benign in their behavior. Based on the presence of stromal invasion, vascular invasion (five tumors), necrosis, high mitotic index, and lymph node metastasis (one tumor), 10 of 97 feline BCTs were considered malignant in one study.79 A case report presented a cat with four concurrent BCTs,81 and there are two cases in the literature deemed malignant BCCs based on the presence of lymphatic vascular invasion in one cat and the presence of pulmonary metastatic disease in the second.82 Nucleomorphometric analysis was able to predict recurrence in one study of 24 cats with BCCs.83 Overall, the likelihood of metastasis with BCCs in cats appears low. Treatment for BCCs is wide surgical excision, which often results in long-term control. Although the data are limited, radiation therapy has been used to treat this tumor, along with subsequent doxorubicin chemotherapy.82 The impact from these treatment options on survival is unknown. Papillomas are benign epidermal proliferative lesions that are often associated with papillomavirus infection.34 They are considered rare in the cat and dog.84 Papillomas typically have an exophytic growth pattern. Another benign variant is the inverted papilloma, which grows into the subcutaneous tissue rather than externally. Cutaneous papillomas are typically found in younger dogs, with an average age of 3.2 years37 (see Figures 1-9 to 1-11). Surgical excision can be curative, and some of these lesions will spontaneously regress.85 In patients with multiple lesions, azithromycin has been shown to be effective.86 In cats, a particular type of papilloma, the fibropapilloma, is seen. These tumors demonstrate a proliferation of mesenchymal cells covered by hyperplastic epithelium.43 Evaluation for papillomavirus demonstrated an apparent nonproductive infection of the mesenchymal cells.87 It has been suggested these feline tumors are more similar to equine sarcoids than papillomas. SCC in situ is defined as a carcinoma that has not penetrated the basement membrane of the epithelium. When it appears in multiple sites, it is also known as Bowen’s carcinoma, Bowenoid carcinoma in situ (BISC), and multicentric papillomavirus-induced SCC. This disease is seen primarily in cats, with only a few reports in dogs.5,48,88–90 Actinic keratosis is the name typically used for SCC in situ that arises as a consequence of UV exposure.76 Differentiation of actinic keratosis from BISC is based on location and histopathologic appearance. Clinically, SCC in situ can present as erosions of the epidermis, proliferations, or crusted plaques. They may be painful on palpation. BISC lesions can occur anywhere on the body—both haired and unhaired skin, areas with and without sun exposure (Figure 18-3). Solitary lesions are unusual.5,89 Actinic keratosis, on the other hand, occurs in lightly haired skin with UV exposure and can occur as a solitary lesion. It is typically accompanied by solar elastosis and fibrosis of the skin, consistent with the effects of chronic UV exposure.76 By definition, carcinoma in situ is not yet invasive, and thus metastasis has not occurred. However, left untreated, carcinoma in situ can progress to invasive carcinoma and put the patient at risk for metastasis. Patients with BISC typically will continue to develop new lesions over time, but metastasis appears to be uncommon.5,48,88–90
Tumors of the Skin and Subcutaneous Tissues
Incidence
Etiology
Viral Factors
Immune Status
Genetic Abnormalities in Skin Cancer
Basal Cell Carcinomas
Squamous Cell Carcinomas
Pathologic Classification of Skin Tumors
Diagnostic Techniques and Work-Up
Treatment and Prognosis for Specific Tumor Types
Epithelial Tumors
Basal Cell Carcinomas
Papillomas
Squamous Cell Carcinoma in Situ
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Tumors of the Skin and Subcutaneous Tissues