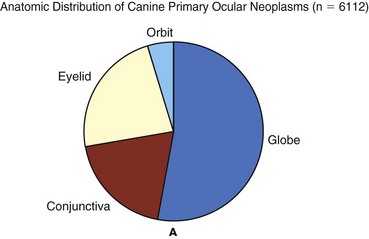
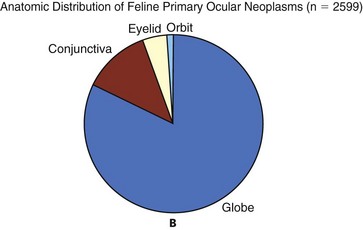
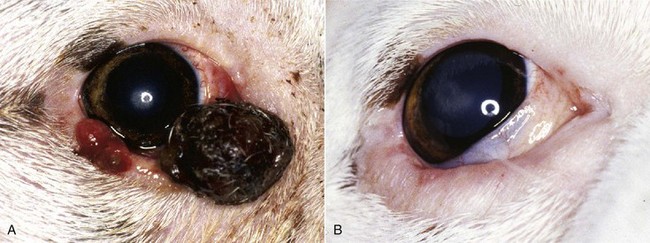
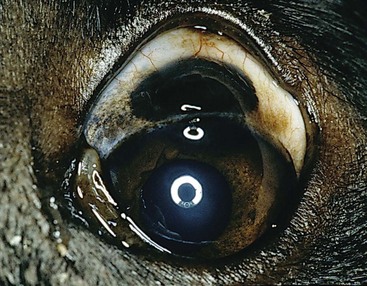
31 Tumors of the eye, orbit, or adnexa can have devastating consequences for an animal’s vision, appearance, and comfort and may be harbingers of potentially life-threatening disease elsewhere in the body. By virtue of their location, even benign ocular tumors may cause blindness and loss of the eye. Although these tumors reportedly affected only 0.87% of all dogs and 0.34% of all cats recorded in the Veterinary Medical Data Base (VMDB) over a 10-year period, their actual frequency is undoubtedly greater because many presumably benign ocular tumors are not histologically examined. Additional insights into the relative frequency of ocular tumors can also be gained by reviewing submissions over several decades to the large ophthalmic pathology database compiled by the Comparative Ocular Pathology Laboratory of Wisconsin (COPLOW) (Figure 31-1). This chapter describes the more common ocular tumors in small animals and also uses the database of the COPLOW laboratory to estimate the relative frequency of various ocular tumors. Benign adenomas and melanomas of the haired skin or eyelid margin make up 81% of eyelid tumors in the COPLOW database and tend to affect old dogs. One study suggests Boxers, collies, Weimaraners, cocker spaniels, and springer spaniels are at greater risk for eyelid neoplasia than the general hospital population,1 and another study suggests that beagles, Siberian huskies, and English setters are at greater risk than mixed-breed dogs.2 Canine juvenile histiocytomas affect the eyelid skin of young to middle-aged dogs1,2 but are relatively uncommon, comprising only 2% of eyelid biopsy samples submitted to COPLOW. Squamous cell carcinoma (SCC) comprises up to two-thirds of feline eyelid and third eyelid tumors and has a predilection for the lower eyelid and medial canthus of white cats.3 Ocular SCC is less frequent in dogs, but in both cats and dogs increased exposure to solar radiation, lack of adnexal pigmentation, and possibly chronic ocular surface irritation are believed to be predisposing factors (Figure 31-2).1,4,5 Vascular endothelial cell tumors of the lateral limbus or the leading edge of the third eyelid constitute 27% of conjunctival tumors in dogs and tend to occur in the nonpigmented conjunctiva in Bassett hounds, springer spaniels and beagles. Melanomas often occur in dogs with a pigmented conjunctiva and are the most common malignant tumor of the canine conjunctiva, making up 17% of conjunctival tumor biopsies. Older (mean = 11 years), female, Weimaraner, and possibly German shepherd/large-breed dogs may be predisposed to conjunctival melanomas.6 Melanomas also have a propensity for the nictitating membrane and superior palpebral conjunctiva.6,7 Ocular viral papillomas compose about 3% of conjunctival tumors and tend to occur in young dogs and are believed to have a papillomavirus etiology (perhaps canine oral papillomavirus).8 Canine squamous papilloma is a benign papillary tumor of unknown etiology and makes up 9% of conjunctival tumor biopsies. Reactive papilloma is seen secondary to other conditions such as meibomian tumors, but they make up 18% of conjunctival tumor biopsies. Corneal tumors have a predilection for the limbus. Sebaceous or meibomian gland adenomas and epitheliomas, papillomas, and melanomas comprise more than 85% of canine eyelid and conjunctival neoplasms, and a substantial majority of these tumors are histologically benign.1,2 Even histologically malignant eyelid tumors in dogs rarely metastasize, although they are more likely to be locally invasive and recur following surgery.1,2 In contrast, most feline eyelid and ocular surface tumors such as SCC are malignant.3 Viral papillomas tend to be well demarcated and superficial, minimally altering deeper tissues. Surgical manipulation has occasionally been associated with dispersal of papillomas throughout the ocular surface.9,10 Papillomas, like histiocytomas, often spontaneously resolve in young dogs, although they may persist in the older dog. SCC may also develop superficially, but following malignant transformation the preinvasive actinic plaque can invade deeper tissues. SCC may spread to regional lymph nodes late in the course of the disease and uncommonly distantly metastasize. SCC of the third eyelid may more readily invade the orbit than corneal or eyelid SCC. Adenocarcinomas of the gland of the third eyelid constitute 13% of conjunctival tumors in dogs. They are variable in morphology and often show moderate infiltrative growth.11 They may mimic prolapse of the gland of the nictitans (“cherry eye”) by appearing as localized, firm, smooth, pink swellings on the posterior surface of the nictitans, but a key differentiating feature is their occurrence in much older dogs (10 to 16 years). Although excision of the grossly visible tumor may initially appear adequate, recurrence is common if the entire gland is not removed, and metastasis, especially to the regional lymph nodes and orbit, is possible.11,12 The natural behavior of conjunctival vascular, melanocytic, and mast cell tumors is poorly understood, in part because they are uncommon. Conjunctival hemangiomas and hemangiosarcomas tend to remain relatively superficial but may recur following simple excision.13–17 Hemangiosarcomas may exhibit a more aggressive course and a primary ocular hemangiosarcoma must be differentiated from a metastatic lesion. However, metastasis of primary conjunctival vascular tumors, even when classified as hemangiosarcomas, appears to be rare.13–17 Feline conjunctival melanomas originate on the bulbar conjunctiva and invade the eyelid.18,19 Melanoma of the conjunctiva in dogs is most often morphologically malignant, but metastatic disease is not common. As in cats, canine conjunctival melanomas have been reported to recur locally following surgical excision in 55% of cases, and at least 17% of the dogs experienced orbital invasion or spread to the regional lymph nodes or lungs.6 Melanomas originating from the palpebral conjunctiva may have greater metastatic potential.6 Mitotic index, cell type, and degree of pigmentation are not useful predictors of malignancy for canine conjunctival melanomas.6 Subconjunctival mast cell tumors make up 4% of conjunctival tumors, but their natural history is poorly understood in part because they are uncommon. Nevertheless, they have been suggested to have a relatively benign course in dogs.20 Vascular tumors are often focal, raised, soft, red masses with visible feeder vessels arising from the surface of the conjunctiva or third eyelid.13–17 SCC of the eyelid, third eyelid, or ocular surface may appear as a focally thickened, roughened, and usually pink-to-red lesion in older animals or, more commonly, as an ulcerated lesion with a protracted course.3–5 In contrast, papillomas in young dogs appear verrucous and usually progress rapidly over weeks to a few months. Nonneoplastic conditions such as nodular granulomatous episcleritis can be mistaken for neoplasia. In addition to a mass lesion, other clinical signs of eyelid or ocular surface tumors may include epiphora, conjunctival vascular injection, mucopurulent ocular discharge, protrusion of the third eyelid, conjunctival/corneal roughening or ulceration, and corneal neovascularization or pigmentation. Occasionally, palpebral conjunctival masses protrude only when their bulk no longer can be accommodated by the space between the eyelid and globe, and very advanced tumors may create exophthalmia or enophthalmia if the orbit is invaded. Large tumors and sebaceous adenomas often have a substantial inflammatory component and may be secondarily infected. Mesenchymal hamartoma appears to have a predisposition for the skin of lateral canthus.21 Tumors involving less than one-fourth to one-third of the length of the eyelid are best treated by a V-plasty (wedge) or four-sided excision.22 The latter technique affords superior apposition of the eyelid margins and wound stability, especially in tumors approaching the one-fourth to one-third limit, because the initial incision is made perpendicular to the eyelid margin rather than obliquely. In general, only one-third to one-fourth of the eyelid in dogs and one-fourth of the eyelid in cats can be removed with these techniques. Antibiotic or antiinflammatory therapy may reduce the size of large tumors that are infected or inflamed so that a wedge or four-sided excision becomes possible. Electrosurgical excision should be avoided because it may result in substantial scarring of the eyelids. Carbon dioxide (CO2) laser ablation may be appropriate for some tumors. Tumors greater than one-fourth to one-third of the eyelid typically require more advanced reconstructive blepharoplasty or utilization of other therapeutic modalities. Some tumors may be responsive to systemic chemotherapy (e.g., lymphoma, mast cell tumors), local infiltration with chemotherapeutic agents such as cisplatin (e.g., SCC),23 and/or local radiation therapy (RT; e.g., SCC). In some cases, these modalities will completely eliminate the tumor or shrink it to the point in which a less extensive surgical procedure can be performed. Reconstructive blepharoplasty, however, is the procedure of choice if surgical cure is a possibility and these other modalities have failed or are unlikely to substantially impact the tumor or if the nature of the tumor indicates extensive margins are required. Cryosurgery is an attractive alternative to extensive blepharoplasty and has been reported to be effective in several canine eyelid tumor types (see Figure 31-2, see also Chapter 10).2,24 It is quick, less technically demanding than reconstructive blepharoplasty, and usually permits preservation of the nasolacrimal puncta and canaliculus. In many old or debilitated patients, cryosurgery can be accomplished with only sedation or local/topical anesthesia. Following pretreatment with dexamethasone (0.1 mg/kg intravenous [IV]), the mass is isolated with a chalazion forceps (if possible) and debulked flush with the lid margin. Using liquid nitrogen and a closed probe that approximates the diameter of the mass as much as possible, a double freeze-thaw is performed so that the iceball extends 3 to 5 mm beyond the visible margins of the mass. Iceballs should overlap in large tumors. Freezing may be repeated a second or third time if complete regression is not achieved following the first session. Substantial postoperative swelling and usually transient depigmentation of the frozen tissue are to be expected. Tumors involving the conjunctiva and third eyelid (especially conjunctival hemangiosarcomas, melanomas, and nictitans adenocarcinomas) are most effectively treated by wide surgical excision—perhaps to the point of exenterating the orbit. If the globe is to be spared, however, excision of the entire nictitans should not be taken lightly because undesirable sequelae such as ocular drying and chronic keratitis frequently result. Bulbar conjunctival tumors move freely and, if small, are generally amenable to excision under only topical anesthesia and perhaps sedation. Cryosurgery may permit the nictitans to be spared in the cases of papillomas and early SCC, or it can be used as an adjunct to excision in advanced canine conjunctival melanomas and SCC.6,24 The prognosis for most canine primary eyelid tumors is excellent, whether treated by excision or cryosurgery. Metastasis is rare, even in histologically malignant primary lid tumors, and recurrence rates are low (approximately 10% to 15%).2 New primary eyelid tumors are not uncommon and must be distinguished from recurrence. Because most eyelid tumors in cats are malignant, the prognosis is not as good as that for dogs, but it is unclear how prognosis correlates with the histologic features. Conjunctival melanomas and nictitans adenocarcinomas frequently recur following partial excision of the nictitans, even if all of the clinically visible tumor was removed.6 Conjunctival hemangiosarcomas appear to have a good prognosis because total excision may be curative, although recurrence and loss of the eye is still possible.13–17 Limbal melanomas are typically benign, slightly raised, heavily pigmented masses originating from melanocytes in the sclera or subconjunctival connective tissue (Figure 31-3).25–30 They comprise 3.5% of all canine ocular tumors and 1% of feline ocular tumor submissions to COPLOW. The majority of these slow-growing tumors originate in the superior limbal region, suggesting exposure to solar radiation may be a risk factor.13 Affected dogs average 5 to 6 years old (cats 8+ years), and a female sex and German shepherd, golden retriever and Labrador retriever breed predilection has been inconsistently reported.25–29 Confirmed metastasis has not been reported in dogs or cats and mitotic figures are rarely encountered; although in one study, two of four cats also had feline leukemia virus (FeLV)-associated lymphoma or leukemia, and a third cat had a second intraocular pigmented mass unassociated with the limbal tumor.29 Lightly pigmented spindle cells capable of division are seen histologically, but the dominant cell is presumably a hypermature spindle cell that is large, round, pigment laden, and benign.26 These masses are often only incidentally noted and the clinical signs are typically minimal, although local corneal invasion, epiphora, and mild conjunctival irritation may be seen.27,28 Differential diagnoses include conjunctival melanoma, invasive uveal melanoma, metastatic melanoma, and staphyloma or coloboma. Gonioscopy aids in differentiating invasive intraocular tumors from limbal melanomas. Therapy should be considered if the tumor has invaded the eye or if growth is rapid. Given its benign nature and usually slow growth rate (imperceptible growth over 18 months has been described), observation alone may be appropriate in older dogs. If intervention is required, lamellar keratectomy/sclerectomy with graft placement is often curative.31 Beta-irradiation and cryosurgery have been used as adjuncts to surgery.31,32 Cryosurgery alone or laser photocoagulation30 has also been described as effective means of treatment. Regrowth following local surgical excision occurs in approximately 30% of patients, but 2 to 3 years may pass before the anterior chamber is invaded and enucleation is required.26–28 The addition of adjunctive therapy such as cryotherapy or beta-irradiation substantially reduces the risk of recurrence following local excision.31,32 Enucleation is curative and indicated if painful intraocular disease is present.26 Canine Anterior Uveal Melanomas In the Armed Forces Institute of Pathology collection of tumors from the canine eye, orbit, and adnexa, intraocular melanomas constitute 12%, other primary intraocular tumors 14%, and metastatic intraocular neoplasms 9%.33 In the COPLOW archive, uveal melanocytic tumors make up 27% of all ocular tumor submissions. Any age is at risk, but most affected dogs are older than 7 years of age, and breed or sex predilections are inconsistent.26 Approximately 78% of canine intraocular melanomas are benign, and 93% arise from the iris or ciliary body (Figure 31-4).26,27,34 The most clinically useful classification scheme classifies these tumors simply as melanocytoma (benign) and melanoma (potentially malignant) based on nuclear features of the tumor cells—with mitotic rate being the most important.26 Benign tumors have fewer than 2 mitotic figures/10 HPF (mitotic index), and malignant tumors demonstrate nuclear pleomorphism and a mitotic index of at least 4 and often more than 30. Destruction of the eye is not by itself sufficient for a diagnosis of malignancy.26 The overall rate of metastasis of intraocular melanomas is approximately 4%34 and usually occurs via the hematogenous route. Local spread along ocular vessels and nerves or via direct penetration of the sclera or cornea also occurs. Benign tumors tend to be more darkly pigmented than malignant tumors.26 Circumscribed, nevus-like pigmented iridal growths have been described in young dogs (7 months to 2 years old).27 The natural history of these lesions is variable because enlargement may not occur over several years.27,33 Some, however, are capable of rapid growth, but to date all are clinically and histologically benign. Ocular melanosis of Cairn terriers resembles feline diffuse iris melanoma in some respects.35 This disorder is probably an autosomal-dominant condition with a variable age of onset and rate of progression. It results in a thickening and pigmentation of the iris, release of pigment into the aqueous, pigment deposition in the sclera/episclera, and to a lesser extent posterior segment pigment deposition. Secondary glaucoma is common, and overt uveal melanocytic neoplasia occurs in a small percentage of dogs.35 Common presentations of intraocular tumors include a visible intraocular or scleral mass, glaucoma, hyphema, anterior uveitis, or extrabulbar spread, or they can be an incidental finding during an ophthalmic examination.26,34 Because glaucoma or hyphema are often the only overtly visible clinical signs,26,34 intraocular neoplasia should always be considered in animals with hyphema, glaucoma, or both when there is no history of trauma or coagulopathy. Small masses frequently create few symptoms other than pupillary distortion. Pigmentation is variable and not a reliable indicator of tumor type. Canine primary intraocular tumors are generally carefully observed. Digital photographs are a valuable aid in assessing progression. Enucleation is advised if there is concern about malignancy or if complications such as intractable uveitis or secondary glaucoma occur.36 In the COPLOW collection, 14% of canine globes with glaucoma that are removed also had melanoma. The low risk of metastasis and unproved efficacy of enucleation at preventing metastasis in the few malignant tumors that have been reported, however, make it difficult to automatically advise enucleation of normotensive, noninflamed, visual eyes.26 Isolated primary masses involving only the iris or a portion of the ciliary body may be amenable to local resection by sector iridectomy/cyclectomy in order to preserve the eye and vision.27,33 These intraocular procedures, however, require an accomplished ophthalmic surgeon and often have unsatisfactory long-term results. Transscleral and transcorneal Nd:YAG or diode laser therapy has induced remission in some small- to moderate-sized primary intraocular tumors.36,37 Specialized goniolens may also allow laser treatment of masses that have invaded into the iridocorneal angle. Although the results were variable, perhaps because these tumors varied histologically in nature, laser therapy holds promise for the palliation or potential cure of a number of intraocular tumor types while also preserving vision. Metastasis was not observed following this procedure, although this obviously remains a risk when the tumor is malignant.36
Ocular Tumors
Tumors of the Eyelids, Third Eyelid, Conjunctiva, and Ocular Surface
Pathology and Natural Behavior
History and Clinical Signs
Therapy
Prognosis
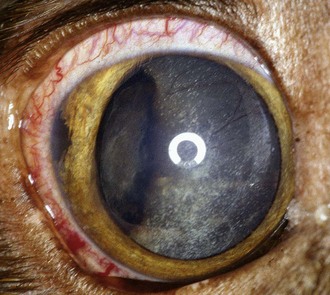
Limbal (Epibulbar) Melanomas
Primary Ocular Tumors
Incidence and Risk Factors
Pathology and Natural Behavior
History and Clinical Signs
Therapy
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Ocular Tumors