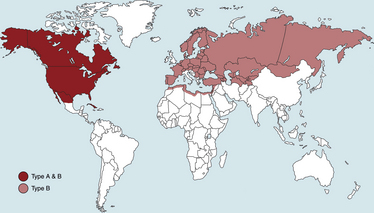
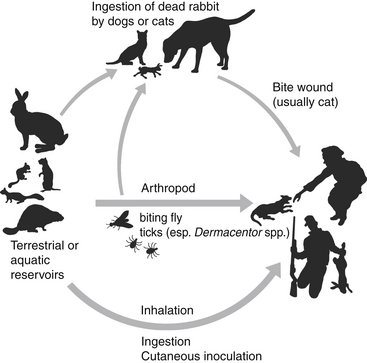
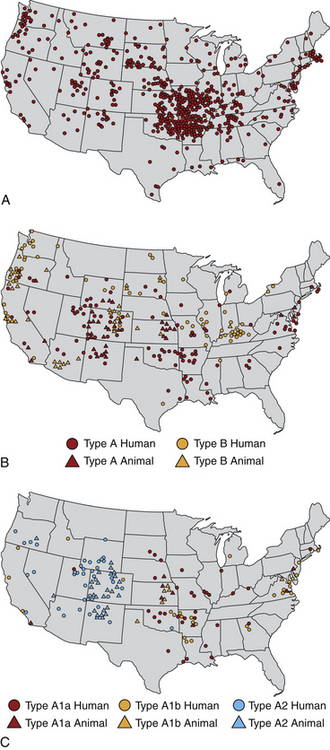
Chapter 56 F. tularensis subsp. tularensis has the greatest degree of virulence for humans and predominates in North America, whereas the less virulent subspecies holarctica predominates in Europe. Additional genetic variation within type A and type B subpopulations of F. tularensis correlates with geographic distribution of cases and the severity of disease.2 Molecular subtyping has further divided type A into subpopulations A1a, A1b, A2a, and A2b. The highest mortality in humans, 24%, results from infections by A1b.3 Tularemia occurs primarily in the Northern Hemisphere, especially between 30 degrees and 71 degrees of latitude (Figure 56-1). Human disease caused by a subspecies novicida–like organism was reported from the Northern Territory in Australia in 2003.4 Even in North America, where F. tularensis subsp. tularensis predominates, tularemia is a rare disease, and most human cases occur in the central and north-central United States, especially Arkansas, Missouri, Kansas, South Dakota, and Oklahoma, with an additional focus in Massachusetts (especially Martha’s Vineyard) (Figure 56-2). Despite its rarity, tularemia is a reportable disease in the United States because F. tularensis is considered a potential agent of bioterrorism. In western Europe, tularemia is endemic in Scandinavia but has emerged in new parts of Sweden and is increasingly reported in humans from more southern locations, such as Germany, France, and Spain.5 Tularemia has been absent from the United Kingdom, with the exception of travel-related disease. It also occurs in Russia and Japan.6 FIGURE 56-2 Geographic distribution of tularemia in the USA. Compare this with the geographic distribution of plague (Figure 55-3). A, Overall distribution of human tularemia cases. B, Distribution of type A and type B isolates from humans and animals. C, Distribution of isolates from humans and animals by molecular subtype. Note that the geographic distribution of human type A cases follows the distribution of subtype A1, the most virulent subtype being A1b. (A and B modified from Kugeler KJ, Mead PS, Janusz AM, et al. Molecular epidemiology of Francisella tularensis in the United States. Clin Infect Dis 2009;48:863-870.) Canine and feline disease due to F. tularensis has only been reported from the United States. Young adult cats and dogs are usually affected. As with plague, cats are more susceptible than dogs. Cases are reported most often from Oklahoma7–9 and Kansas,10 but also from other locations that include Montana,11 Missouri,12 Massachusetts,13 central Virginia,14 and eastern Oregon,15 which mirrors the distribution in humans. Tularemia is likely underdiagnosed in cats and dogs because clinical signs are nonspecific. Many cats with tularemia are diagnosed with tularemia at necropsy.7,10 The seroprevalence in pet cats from Connecticut and New York state was 12%.16 F. tularensis is maintained in the environment by a huge variety of terrestrial and aquatic mammals, which include rabbits, hares, ground squirrels, prairie dogs, lemmings, voles, beavers, muskrats, water rats, and other rodents.6 It often causes severe disease in these species, although subclinical carriage may also occur. It can be transmitted by arthropod bites, inhalation or ingestion of the organism, and direct skin contact with infected tissues (probably when there are minute breaks in the skin) (Figure 56-3). Inhalation of as few as 10 organisms can cause human disease, whereas larger numbers of organisms (108) must be ingested for disease to occur. Cats and dogs become infected when they hunt rodents or lagomorphs; a history of rabbit ingestion was most often described in association with the few reported feline and canine cases.7–9 In the western United States (Utah, Nevada, and California), the most common arthropod vectors are biting flies, whereas ticks (especially Dermacentor ticks and Amblyomma americanum) are the most common vectors east of the Rocky Mountains. In Europe, Dermacentor ticks and Ixodes ricinus are the main tick vectors; mosquitos are important vectors in Scandinavia.17 F. tularensis is well known for its ability to survive up to several months in fresh water sources, and survival in brackish water has also been demonstrated.18 Survival and replication of F. tularensis within free-living amoebae such as Hartmannella vermiformis may contribute to the organism’s ability to persist in water sources.19 The organism can also survive for years in frozen rabbit meat. The clinical manifestations of tularemia depend on the virulence of the infecting strain, the route of inoculation, and host immunity. Although tularemia may rapidly progress to death in cats, protracted illness has also been described.15 F. tularensis survives in macrophages through evasion of cellular destruction by phagosomes and inhibition of the respiratory burst. Several different virulence factors of F. tularensis have been identified, which include bacterial lipopolysaccharide (LPS), pili, a capsule, and a series of acid phosphatase enzymes. The endotoxic activity of the LPS of F. tularensis is 1000-fold less potent than that of Escherichia coli.20,21 Production of acid phosphatase enzymes may be key to the organism’s ability to inhibit the respiratory burst and survive in macrophages.22 Many stages of the organism’s intracellular lifestyle depend on a pathogenicity island (group of virulence genes) known as the Francisella pathogenicity island. For the first few days after cutaneous inoculation, F. tularensis replicates locally and can produce a papule that may then ulcerate. It then disseminates to local lymph nodes, which may be followed by bacteremia and sepsis, with infection of systemic, primarily reticuloendothelial tissues. The organism invades not only macrophages but also nonphagocytic cells such as hepatocytes and alveolar epithelial cells,23 with induction of a profound inflammatory response and necrosis. Incubation periods that range from 1 to 5 days have been suspected in naturally occurring tularemia in dogs and cats, but longer incubation periods (up to 21 days) can occur in humans. Clinical signs typically manifest as a sudden onset of fever, lethargy, and inappetence. Other manifestations in affected cats have included a thin body condition, icterus, hepatomegaly, vomiting, dehydration, peripheral and/or abdominal lymphadenomegaly, splenomegaly, and oral and/or lingual ulceration. Hypothermia and bradycardia can occur in moribund cats7; one cat had seizures and was nonresponsive.14 A single cat with localized cutaneous tularemia had a chronic (1-year duration) draining skin lesion in the region of (but not involving) the mandibular lymph nodes, in the absence of systemic signs.15 In the rare reports of naturally affected dogs, clinical signs have included fever, anorexia, and peripheral lymphadenopathy.8,24 One dog also had bilateral mucoid ocular discharge and mild conjunctival hyperemia,8 and another had tonsillitis.24 Physical examination in cats with tularemia may reveal lethargy, fever (up to 105.1°F [40.5°C]) or hypothermia, a thin body condition, and dehydration.7,9,11–13 Ulceration of the oral mucous membranes or tongue may be present.7,13 Icterus may also occur.25 Moribund cats may be hypothermic and bradycardic and may have weak pulse quality. Mild to marked peripheral lymphadenopathy is present in most but not all cats. Lymphadenopathy may be restricted to the lymph nodes of the head and neck13 or may be generalized.7,9 Abdominal palpation may reveal hepatomegaly or splenomegaly, or abdominal lymph nodes may be palpable. Fever, lethargy, peripheral lymphadenopathy, mucoid ocular discharge, and tonsillitis may be present in dogs.8,24 Tularemia should be considered in cats and dogs with lymphadenopathy and fever that have a history of residence in or recent travel to an endemic area, as well as contact with or predation of wild mammals (especially lagomorphs). Plague is the major differential diagnosis of zoonotic importance in the southwestern United States (Table 56-1). Ultimately, the diagnosis can be confirmed by culture, PCR, and/or serologic testing. With the exception of PCR, access to approved laboratories that practice biosafety level 3 procedures is required to perform these tests when tularemia is suspected. Local public health authorities should be contacted to report suspicion for the disease and for information on recommended testing facilities. TABLE 56-1 Differences and Similarities between Plague and Tularemia in Cats and Dogs in the United States Complete Blood Count, Biochemistry Profile, and Urinalysis Leukocyte counts in a limited number of cats with tularemia have been normal, decreased (as low as 1300 cells/µL), or increased with evidence of neutrophil toxicity.7,9,13,14 Moderate to marked bandemia may be present.9,13 Thrombocytopenia is a common finding (as low as 41,000 platelets/µL).7,9 The serum biochemistry panel may show hypoglycemia, azotemia, and electrolyte abnormalities, increased liver enzyme activities, and hyperbilirubinemia (up to 7.6 mg/dL).7,9,14 Bilirubinuria and hemoglobinuria may be present.7 Mature neutrophilia, lymphopenia, and increased serum ALP activity were present in a dog with tularemia.8 In human patients, abnormalities include thrombocytopenia, elevated serum transaminase activities, increased serum creatine kinase activity, myoglobinuria, and pyuria.26
Tularemia
Etiologic Agent and Epidemiology
Clinical Features
Physical Examination Findings
Diagnosis
Plague
Tularemia
Organism
Gram-negative bacillus (Enterobacteriaceae). Survives relatively poorly in the environment. Grows on routine culture media used for gram-negative bacteria.
Gram-negative coccobacillus (Francisellaceae). Can persist in the environment and water sources for months. Fastidious growth in culture; often requires special media and prolonged incubation.
Major Arthropod vectors
Diverse variety of rodent and rabbit fleas
Dermacentor spp. and Amblyomma americanum ticks
Geographic location
Southwestern United States
Western, southwestern, central, and eastern United States
Affected hosts
Cats > dogs
Cats > dogs
Usual history
Rodent contact or exposure to rodent fleas
Rodent and especially rabbit contact; ingestion often reported
Clinical signs
Acute disease with high fever and lymphadenopathy, most often of the mandibular and/or retropharyngeal lymph nodes. Hyperbilirubinemia and icterus do not appear to be common in affected cats.
Acute or more chronic disease (sometimes associated with a thin body condition) with oral ulceration, lymphadenopathy of the mandibular and/or retropharyngeal lymph nodes, or generalized lymphadenopathy and abdominal organomegaly. Hyperbilirubinemia is frequent in affected cats, and icterus has been described.
Lymph node aspirate cytology
Neutrophilic inflammation; monomorphic population of bipolar staining bacilli often present
Lymphoid reactivity or neutrophilic to pyogranulomatous inflammation and necrosis; bacteria often not visualized
Antimicrobial drugs
Aminoglycosides, doxycycline, chloramphenicol, trimethoprim-sulfamethoxazole
Aminoglycosides are the treatment of choice. Doxycycline, chloramphenicol, and fluoroquinolones may be effective but may be followed by relapse.
Zoonotic potential
Exposure to cats with pneumonic plague can lead to human infection; bites and scratches have also been implicated. Human pneumonic plague usually results from exposure to infected cats and can progress to death within a few days. Exposed humans may require prophylaxis with doxycycline.
Most often follows bites from affected cats, less often scratches. Course of disease longer and mortality may be lower than occurs with pneumonic plague. Medical attention should be sought if bites or scratches occur.
Laboratory Abnormalities
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Tularemia
Only gold members can continue reading. Log In or Register to continue