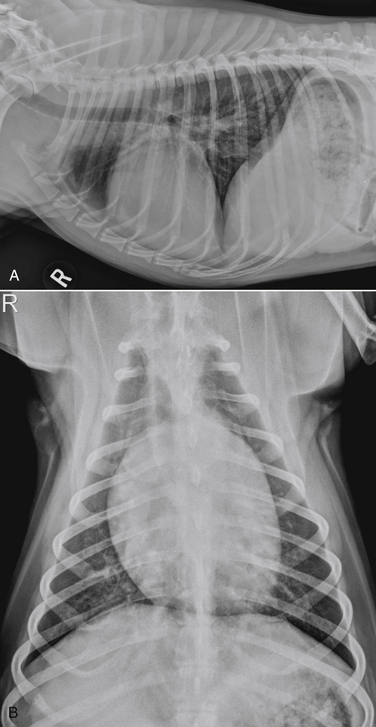
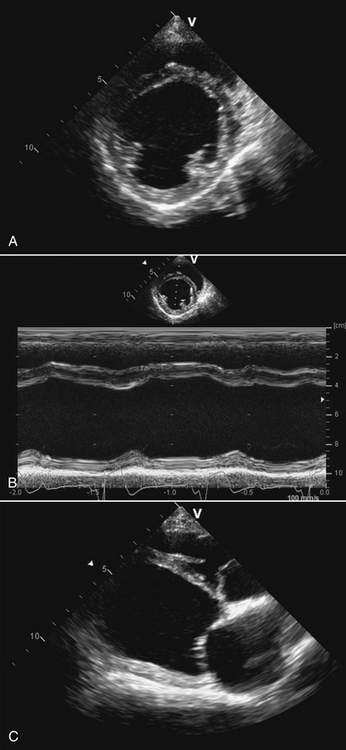
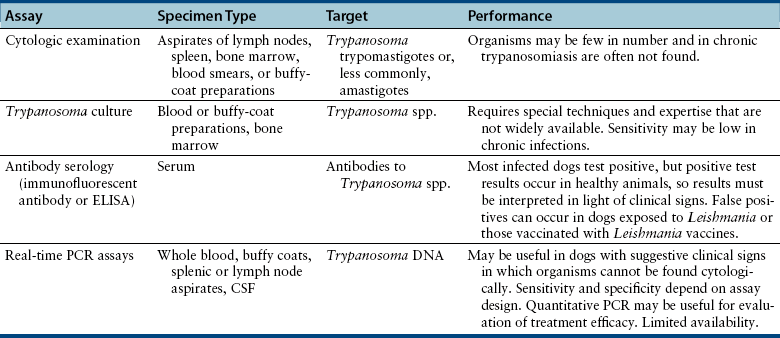
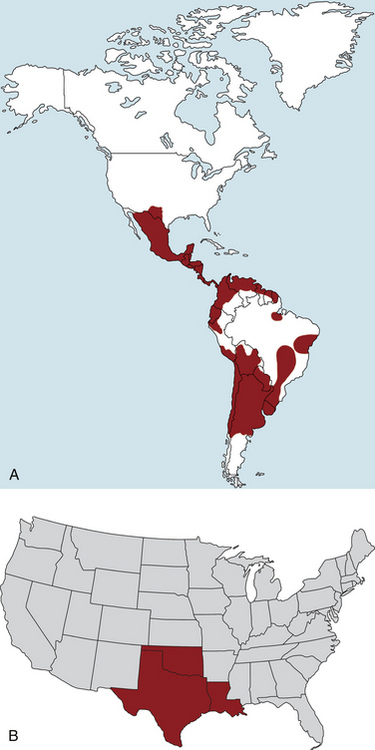
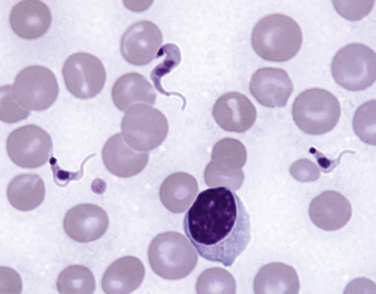
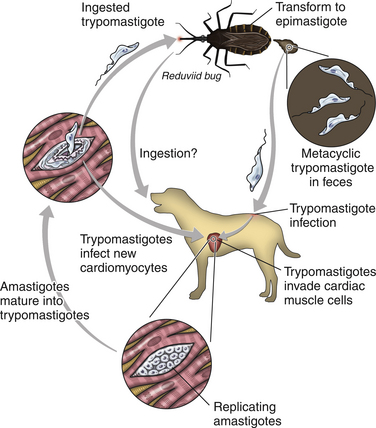
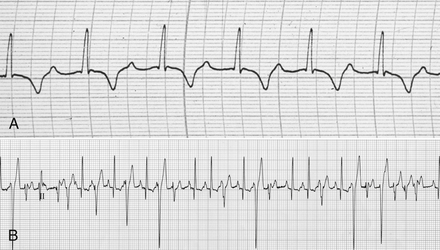
Chapter 78 Chagas’ disease, or American trypanosomiasis, is caused by the flagellated protozoan Trypanosoma cruzi. In North America, disease in dogs usually manifests as cardiac disease typified by arrhythmias or myocarditis (acute or chronic), and, rarely, neurologic disease.2–7 However, many infected dogs are subclinically infected for life. Cats can become infected, but disease in cats has not been recognized. Trypanosoma cruzi is an important vector-borne zoonosis in the Americas, particularly South and parts of Central America, and is the leading cause of dilated cardiomyopathy in humans.8 Within the United States, most cases in dogs occur in Texas and especially involve working dogs that reside in southeastern Texas (Figure 78-1).9–11 A few affected dogs have been reported from other southern states,5,6,12–14 and as far north as Missouri.15 The seroprevalence in dogs from Louisiana and Texas has generally been in the range of 12% to 22%, with seroprevalences of <10% in other states where canine disease has been identified.16 Canine Chagas’ disease is of importance to veterinary practitioners because it can be difficult to diagnose, is a potential zoonosis, and there is a lack of therapeutic options. FIGURE 78-1 A, Approximate distribution of Chagas’ disease in humans in the Americas. Insect-transmitted human Chagas’ disease has also been rarely reported in Tennessee, California and Louisiana. B, States within the United States where Chagas’ disease is most prevalent in dogs. Disease in Texas primarily occurs in the southeast. Occasional cases have been reported in other southern states and as far north as Virginia and Missouri. Trypanosoma cruzi exists in three morphologic forms. The form that circulates freely in the host’s peripheral blood is the trypomastigote. This form is 15 to 20 µm long, with a flattened spindle-shaped body and a centrally placed vesicular nucleus. A single flagellum originates near a large, subterminal kinetoplast (situated posterior to the nucleus) and passes along the body to project anteriorly (Figure 78-2). The host intracellular or amastigote form is approximately 1.5 to 4.0 µm in diameter, is roughly spheroid, and contains both a nucleus and a rod-like kinetoplast. A small flagellum is present, but this is rarely obvious under light microscopy. The third morphologic form, the epimastigote, is found in the arthropod vector, a reduviid or “kissing bug” (subfamily Triatomae). Epimastigotes are flagellated and spindle shaped with the kinetoplast situated anterior to the nucleus. When a vector is involved in transmission, infection occurs when trypomastigotes are deposited in the triatomine’s feces at the insect bite site. This is the main mode of transmission to humans in South America. FIGURE 78-2 Trypomastigotes of Trypanosoma cruzi in a blood smear of a dog. Wright-Giemsa stain, 1000× oil magnification. (From Barr SC. Canine Chagas’ disease (American trypanosomiasis) in North America. Vet Clin North Am. 2009;39:1055-1064.) Transmission of T. cruzi in endemic countries depends on the confluence of vectors, reservoirs, parasites, and hosts (both people and animals) in a single habitat. Only three Triatomae species (Triatoma infestans [the main vector in South America], Triatoma dimidiata, and Rhodnius prolixus) that feed on man in endemic regions in South America display the appropriate behavior that enables them to transmit T. cruzi effectively. These species feed on blood from both people and domestic reservoir mammals (dog, cat, guinea pigs), reproduce prolifically while cohabiting close to people, and defecate soon after taking a blood meal, meaning that they are usually still on the host near the bite wound when they defecate.17 Infection rates in these vectors can be as high as 100% south of the equator. By contrast, domestic transmission cycles probably do not occur in the United States, except in areas of southeastern Texas where there is evidence to suggest that the dog can be involved in domestic transmission cycles involving vectors and man.18 Generally, though, the two principal vectors in the United States (Triatoma protracta and Triatoma sanguisuga) have low infection rates (20%), display different feeding habits, and defecate about 20 minutes after feeding, often when they have long fled the host.19 As a result, it is more likely that dogs in North America become infected when they eat infected triatomines or their excreta. Certainly, opossums20 and armadillos21 become infected by this route, and outbreaks have occurred in humans following ingestion of contaminated food or drinks.22 Blood transfusion and transplacental transmission can also occur, and infection after ingestion of infected milk from lactating bitches has been proposed.6 Whether ingestion of reservoir hosts leads to transmission is unclear. Within the United States, the principal wildlife reservoir hosts of T. cruzi in the eastern seaboard states south from Maryland and in most other southern states (Texas, Louisiana, and Oklahoma, to name a few) are opossums and raccoons. Armadillos can also be infected.23 Various mouse, squirrel, and rat species are the main reservoir hosts in New Mexico and California.24 Isolates of T. cruzi from vectors and animal reservoirs in North America are less pathogenic in mice than South American isolates.23,25 Experimental inoculation of T. cruzi isolates from opossums and armadillos into dogs produces a disease consistent with naturally acquired acute and chronic canine trypanosomiasis, so it is likely that dogs in nature are infected with the same isolates as these wildlife hosts.2–6 Six genotypes of T. cruzi have been identified (TcI through TcVI, also known as discrete typing units or DTUs), which appear to differ in their vector preferences and the extent to which they cause disease.26 After infection, trypomastigotes enter macrophages, transform into amastigotes, and multiply by binary fission (Figure 78-3). Alternatively, they spread hematogenously from the site of infection, infect myocardiocytes, transform into amastigotes, multiply, and transform back into trypomastigotes. The host cell then ruptures and trypomastigotes are released back into circulation. Parasitemia in dogs appears as early as 3 days postinfection (DPI), peaks at 17 DPI, and is cytologically undetectable (subpatent infection) by 30 DPI.2 Clinical signs of acute myocarditis, should they occur, develop about 14 DPI with recovery occurring around 28 DPI.2 Rapid intracellular multiplication ensures a rapid rise in parasitemia before effective immunity develops. Parasitemia steadily rises as more and more intracellular multiplication cycles add to the number of circulating trypomastigotes. The vector becomes infected when it ingests circulating trypomastigotes, which transform into epimastigotes and multiply by binary fission. Transformation of the epimastigotes back into trypomastigotes occurs in the vector’s hindgut before the trypomastigotes are passed in the feces. FIGURE 78-3 Life cycle of Trypanosoma cruzi. Dogs are infected when a triatome (reduviid bug) defecates at its bite site, and possibly when dogs ingest infected triatomes. Other routes of transmission may also predominate in the United States (see text). Trypomastigotes infect cardiomyocytes (as well as other tissues) and form amastigotes, which replicate locally, leading to myocarditis. As in humans, there are three phases of Chagas’ myocarditis in dogs: acute, indeterminate (or latent), and chronic.2–6 Acute myocarditis results from cell damage and inflammation as trypomastigotes rupture from myocardiocytes. Lethargy, fever, inappetence, generalized lymphadenopathy, slow capillary refill time with pale mucus membranes, and, in some cases, splenomegaly and hepatomegaly are the main signs in puppies. Diarrhea can also occur. In dogs over 6 months of age, parasitemia develops more slowly and clinical signs are often much less severe or not apparent at all. Sudden death, presumably from cardiac muscle failure or conduction system failures leading to malignant arrhythmias, is not a common occurrence. Although less common than signs referable to cardiac abnormalities, neurologic signs referable to meningoencephalitis (as a direct result of parasitic invasion of the neurologic system) may also occur and include weakness, pelvic limb ataxia, and exaggerated spinal reflexes suggestive of distemper. Humans with Chagas’ disease can also develop severe megaesophagus or megacolon due to parasympathetic denervation,22 but these have not been described in dogs. Dogs that survive the acute phase enter a prolonged indeterminate phase typified by a lack of clinical signs. Parasitemia becomes cytologically undetectable at about 30 DPI, although it can still be demonstrated by blood culture. The ECG is usually normal during this phase, although ventricular arrhythmias can be induced by exercise.3 Although not all dogs progress to develop chronic disease, some develop chronic myocarditis with cardiac dilatation over the subsequent 8 to 36 months.2,3 ECG abnormalities become more prevalent in these dogs and may even result in sudden death. Clinical signs referable to right-sided and eventually, in some, left-sided heart failure occur and can include exercise intolerance, pulse deficits, ascites, pleural effusion, hepatomegaly, and jugular venous congestion.2 Chronic Chagas’ myocarditis is clinically indistinguishable from dilated cardiomyopathy of large breed dogs.4–6 The pathogenesis of the cardiomyopathy is unknown, but proposed mechanisms include damage to myocardiocytes or the autonomic nervous system by immune-mediated mechanisms or toxic parasitic products, and/or microvascular disease coupled with platelet dysfunction.27,28 Cardiac dilatation occurs when fibrosis no longer permits efficient compensatory hypertrophy.27,29 Some T. cruzi isolates that infect dogs in the United States are not pathogenic but produce a marked serologic response and a low-level parasitemia following immunosuppression.2,30 Reactivation of infection with immunosuppression can occur in human patients.22 The most common physical examination findings in puppies with Chagas’ disease are lethargy, generalized lymphadenomegaly, splenomegaly, pallor, and arrhythmias. Generalized subcutaneous edema has also been reported.31 Dogs with chronic Chagas’ disease may show signs of right- and/or left-sided congestive heart failure, with tachypnea, ascites, or jugular pulses. Cardiac murmurs and/or arrhythmias may be detected on auscultation of the heart. During the acute T. cruzi infection, the CBC of some dogs may reveal anemia, leukocytosis due to a neutrophilia, eosinophilia, monocytosis, and lymphocytosis.32,33 Thrombocytopenia, leukopenia, and/or lymphopenia have also been documented.16 Dogs in the chronic phase of infection may have no hematologic abnormalities. Serum ALT activity, AST activity, and creatinine and urea nitrogen concentrations can be elevated in dogs with T. cruzi infection, especially those that are at risk of death from severe acute myocarditis. Rarely, moderate to severe hypoalbuminemia with or without hyperglobulinemia occurs in acutely affected dogs.16,31 Serum troponin I levels rise slowly in infected dogs and peak at around 10 to 30 mg/mL by 21 DPI. Serum troponin I levels are elevated in dogs infected after 6 months of age but usually not to such high levels. The ECG of dogs with severe Chagas’ myocarditis may show sinus tachycardia, decreased R-wave amplitude, axis shifts, T-wave inversion, ventricular arrhythmias, and conduction abnormalities, including first-, second-, and third-degree atrioventricular block and right bundle branch block (Figure 78-4). FIGURE 78-4 Electrocardiographic findings in dogs with Chagas’ disease. A, Second-degree heart block and depressed QRS complexes. (From Barr SC. Canine Chagas’ disease (American trypanosomiasis) in North America. Vet Clin North Am. 2009;39:1055-1064.) B, Multiform ventricular premature complexes in an 11-year-old, neutered male Labrador retriever presenting for collapse. Recording speed 25 mm/s, sensitivity 10 mm/mV. Plain thoracic radiographs in dogs with chronic Chagas’ myocarditis may reveal cardiomegaly or evidence of congestive heart failure (pulmonary edema or pleural effusion) (Figure 78-5). Echocardiograms of puppies with acute myocarditis are usually within normal limits. Echocardiographic abnormalities in dogs with chronic Chagas’ myocarditis include right ventricular dilation with progression to include a loss of left ventricular function with decreased fractional shortening, reduced ejection fraction, reduced left ventricular free wall thickness, and increased end-systolic volume (Figure 78-6). Diagnostic assays for trypanosomiasis in dogs and cats are described in Table 78-1. During acute disease, trypomastigotes may be detected on blood smear examination (see Figure 78-2). However, only a few parasites may be present on the entire slide, demanding diligent examination, or some form of concentration technique may be used. High-power (400×) examination of the buffy-coat layer from a centrifuged microhematocrit tube may reveal the motile parasites. Examination of a thick-film buffy-coat smear stained with either Wright or Giemsa stains is more sensitive than examination of a blood smear. A highly effective concentration technique involves pelleting trypomastigotes from plasma (obtained by centrifugation of 10 to 50 mL of heparinized blood at 800 × g for 10 minutes) by further centrifugation (8000 × g for 15 minutes). The pellet from the final centrifugation may be examined microscopically after staining or be submitted for PCR analysis or culture. The larger the volume of the blood specimen collected, the greater the likelihood that organisms will be detected. Trypomastigotes may also be found on cytologic examination of lymph node aspirates and in abdominal effusions. Amastigotes were described in the lymph node of one affected dog that were cytologically indistinguishable from those of Leishmania.31 Trypanosoma cruzi can be cultured from blood in liver infusion tryptose (LIT) growth medium. However, several weeks are required before epimastigotes grow in this medium, which is too long to be helpful for making treatment decisions. Although culture is more sensitive than blood smear examination, false-negative results can still occur; in humans the sensitivity of blood culture is estimated to be only 50%.22 Culture of trypanosomes is a specialized procedure that is not routinely available in veterinary diagnostic laboratories. Serology is extremely useful for the diagnosis of Chagas’ disease, especially during the indeterminate and chronic phases when trypomastigotes are very difficult to detect.34 Indirect fluorescent antibody, ELISA, and radioimmunoprecipitation assays are most commonly used. A rapid immunochromatographic dipstick test has also been developed.35 Serologic tests confirm the presence of antibodies to T. cruzi, but most cross-react with antibodies to Leishmania. Further, in rare situations in dogs, the clinical signs of Chagas’ disease and leishmaniasis overlap sufficiently that it is necessary to go to considerable lengths to establish a diagnosis.31 Therefore a detailed history of the likelihood of exposure to Leishmania must be known in order to accurately interpret serologic results. A higher antibody titer to Trypanosoma than to Leishmania may support a diagnosis of trypanosomiasis. Positive serology in association with consistent clinical signs is the most common means of diagnosis of Chagas’ disease in dogs. If possible, two different serologic assays should be used to confirm a positive titer, especially in regions of low prevalence. The serum titer usually becomes positive by 21 DPI, when parasitemia is declining, and persists for the life of the animal irrespective of whether clinical signs develop.30
Trypanosomiasis
American Trypanosomiasis
Clinical Features
Physical Examination Findings
Diagnosis
Laboratory Abnormalities
Serum Biochemical Tests
Electrocardiography
Diagnostic Imaging
Sonographic Findings
Microbiologic Tests
Cytologic Examination
Culture
Serologic Diagnosis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Trypanosomiasis
Only gold members can continue reading. Log In or Register to continue