CHAPTER 16 Transtracheal and Bronchoalveolar Washes
Respiratory flush/washes sample the contents of the airways, the trachea, bronchi, and alveolar spaces. These samples can frequently provide clinically useful information of the pulmonary disease process, and may also provide definitive diagnosis in some patients. Pulmonary disease is frequently defined by the area that it affects (e.g., bronchitis) or by the changes that may occur as a result of the disease process (e.g., bronchiectasis); however, the underlying pathology may be variable with these disease presentations, and cytology and culture of a lower respiratory tract sample may be helpful in determining the etiology.1,2,3 In pathologies that solely involve abnormal structure or function of the airways, or in diseases that do not have direct airway involvement, which may include some primary or metastatic neoplasias, the information obtained from a flush/wash sample may be limited.4
Tracheal wash/bronchoalveolar lavage (TW/BAL) samples are quick, easy, and inexpensive ways to obtain diagnostic samples from the respiratory tree. Although complications are uncommon, subcutaneous emphysema, pneumomediastinum, hemorrhage, resultant hypoxia, needle tract infection, transient hemoptysis, bronchoconstriction, and other complications have been reported.5–7
It is frequently helpful to perform radiography in conjunction with the wash procedure, although radiographic changes may not always be apparent in the early stages of respiratory disease.8,9 Radiography prior to a flush/wash procedure may be invaluable if the disease is focal because this will indicate which lung lobe is most likely to provide a diagnostic yield and allow selective sampling, particularly if bronchoscope guided lavage is used. If the disease is diffuse then sampling of any area of the lung may be representative, although sampling from multiple sites is more likely to provide a diagnostic yield.10
The cell types noted in the sample may be variable depending on the site of sampling (Tables 16-1 and 16-2).
Table 16-1 Lining Cells of the Lower Respiratory Tract that May Be Noted on TW/BAL Sampling
| Airway | Lining cell76 |
|---|---|
| Large airway, trachea, and bronchi | Ciliated columnar epithelium, goblet cell |
| Bronchiole | Columnar to cuboidal epithelium, ciliated to nonciliated Alveoli |
| Alveolus | Type I pneumocyte (not commonly observed on BAL cytology) |
Table 16-2 Average of Mean Percentage Cell Differential of Nonepithelial Populations from a Number of Studies of BAL Samples from Healthy Dogs and Cats

TECHNIQUE OF TRACHEAL WASH AND BRONCHOALVEOLAR LAVAGE
There are a number of reviews of the sampling techniques11–14; however, a brief summary is outlined here.
Transtracheal Sampling
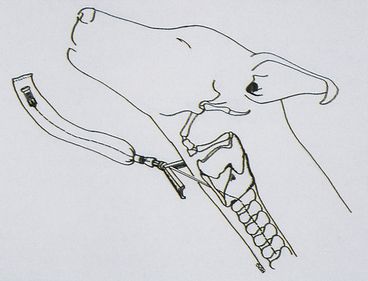
Figure 16-1 Diagrammatic representation of needle place-ment through the cricothyroid ligament of the larynx.
Only a small portion of the injected fluid will be retrieved. The injected fluid remaining in the tracheobronchial tree will be rapidly absorbed and is no cause for concern.5
Maintaining gentle pressure on the puncture site for a few minutes generally inhibits the formation of subcutaneous emphysema.15 Applying mild pressure to the puncture site with a gauze wrap for 12 to 24 hours also helps eliminate the formation of subcutaneous emphysema.
Endotracheal Tube Technique
Alternatively, samples are collected through an endotracheal tube (Figure 16-2). This procedure requires general anesthesia. This technique may be used to obtain either a tracheal sample or bronchoalveolar lavage. For a tracheal sample, the sample catheter extends beyond the end of the endotracheal tube, but does not extend past the carina. The location of the carina is externally assessed as approximately the level of the fourth intercostal space.10 For a blind bronchoalveolar lavage, a sample tube of appropriate size for the patient (e.g., a 16-French polyvinyl chloride stomach tube in a medium- to large-sized dog,17 and a 5-French polypropylene urinary catheter in a cat18) was shown to consistently maintain a snug fit between the external land marks of the seventh and eleventh ribs, so the sample tubing should be a minimum length to reach the level of the eleventh rib.17 Sterile tubing should be used.
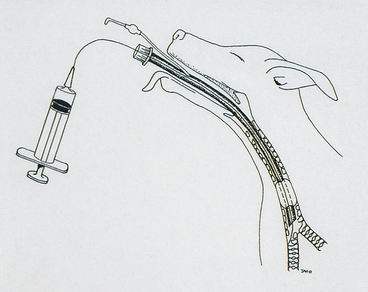
Figure 16-2 Diagrammatic representation of catheter place-ment and TW/BAL collection through an endotracheal tube.
The canine bronchial tree is irregularly branching and has been reviewed in detail.20 From this, briefly, when the patient is orientated in sternal recumbency the entrance to the right principal bronchus appears as almost a direct continuation of the trachea, with the left principal bronchus noted at a more acute angle. The first lobar bronchus on the right is the right cranial lung lobe, in the lateral wall of the bronchus opposite the carina. The next lobar bronchus is the right middle lung lobe, in the ventral floor, usually between the 6 and 8 o’clock positions. The right accessory lobe is located in the ventromedial to medial aspect of the right principal bronchus, just beyond the origin of the middle lobe bronchus, extending in a ventromedial direction. Beyond this bronchus is the lobar bronchus for the right caudal lung lobe. On the left the left cranial lung lobe is accessed ventrolateral to the lateral aspect of the left cranial bronchus. Beyond this, the left principal bronchus becomes the left caudal lung lobe bronchus.
Other measures that may increase fluid retrieval is tilting the head of the patient downward, or rotating the patient with the lavaged lung area uppermost to encourage fluid drainage.25 This may be complicated in larger patients, and the risk of gastric dilation of the volvulus in large, deep-chested dogs may also be a concern with rotation of these patients.
Retrieved fluid should appear foamy if the sampling has been adequate, reported to reflect the presence of surfactant.14
Other techniques that have been reported via bronchoscopic sampling are bronchial brushings and biopsy.15 One study suggests that in some instances bronchial brushing may be a more sensitive test to assess for inflammation, although in one patient BAL was the more sensitive test26; however, the criteria to determine what constitutes inflammation from these types of samples alone is not clearly defined.
Bronchoscopy may also be used for treatment, as in the removal of tracheobronchial foreign bodies.27 Therapeutic bronchoalveolar lavage has also been described in one dog affected by pulmonary alveolar proteinosis.28
SAMPLE SUBMISSION
Several studies in healthy patients have shown that there are no significant differences between different lobes of the lung lavaged either in overall cell numbers or differential counts.19,29 Therefore increases of cells will be interpreted similarly no matter which area of the lung the samples are derived from. The first aliquot is reported to have fewer epithelial cells and higher numbers of polymorphonuclear cells, and some authors recommend discarding the first aliquot, although if combined with subsequent aliquots it is unlikely to significantly affect clinical interpretation.22
It is recommended to prepare fresh smears at the time of sample collection, within 30 minutes29 because cell morphology is not well preserved in TW/BAL samples. A direct smear of turbid fluid, or if mucous flecks are noted grossly, a smear of mucus material and additional cytocentrifuged preparations are likely to provide the most information from the sample. Cytocentrifugation has been reported to affect the cell populations, particularly reducing the number of small lymphocytes present.30
Recommendations for samples submitted to the laboratory are noted in Box 16-1. Guidelines for preparing smears from fluids are presented in Chapter 1.
If the TW/BAL is deemed unacceptable because of oropharyngeal contamination (or for any other reason) and is to be repeated, it should be repeated either immediately or after 48 hours. Even though a sterile saline solution is used for the wash, it induces a neutrophilic response that peaks about 24 hours after washing. If a TW/BAL is performed the next day (i.e., 24 hours after the first wash), an inflammatory response will be present and it may be difficult to tell whether it is secondary to the prior wash or because of an inflammatory lung disease.31–33 However, there may be no significant difference in samples collected 48 hours apart.23,31,33 If a contaminated wash is obtained, waiting at least 48 hours to re-collect a TW/BAL is ideal because it allows the lungs time to clear the oropharyngeal contaminants. Sometimes, however, such a delay is not practical. Although some contaminants from the previous wash may be collected, if the TW/BAL is repeated immediately, the amount of oropharyngeal contamination should be minimal, and this may be preferable to waiting 48 hours.
CELL COUNTS
Cell counts are difficult to perform on TW/BAL fluids because of the mucous content, and the dilution factor may be variable.34 The method of obtaining cell counts is also varied in many studies of TW/BAL in dogs and cats, so values may not be directly comparable, and diagnostic significance is often difficult to determine. Cell counts are recommended by some authors to determine whether an adequate sample has been obtained and to assess whether resampling is necessary. However, this may not be easily applicable in the practice setting. Qualitative estimates (normal or increased) of cellularity can be done on stained sediment smears and may be useful.
CYTOLOGIC EVALUATION
Mucus
A small amount of mucus may be present in TW/BAL from clinically normal dogs and cats. Mucus appears as amorphous sheets ranging from blue to pink or as homogeneous strands that are frequently twisted or whorled5,25 (Figure 16-3; see also Figures 16-6, 16-11, 16-12, 16-16, and 16-17). A granular appearance of the mucus is frequently associated with increased cellularity.5 Inflammation, irritation, or upper airway damage, which may be a result of chronic airway disease, may result in increased numbers of goblet cells, and an increased amount of mucous is generally present, possibly with altered mucous properties.5,31,35 In inflammatory conditions, mucus usually stains eosinophilic because of the incorporation of inflammatory proteins and material from lysed cells.5
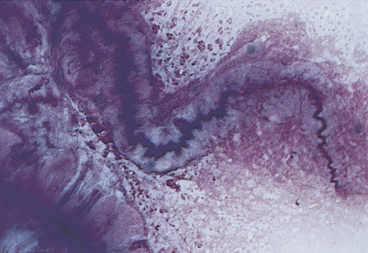
Curschmann’s spirals (see Figure 16-3) are mucous casts of small bronchioles that appear as spiral, twisted masses of mucus that may have perpendicular radiations, giving them a test tube-brush-like appearance.4 They may be seen in TW/BAL from patients with any disorder that results in chronic, excessive production of mucous and are an indication of bronchiolar obstruction.
Cell Types
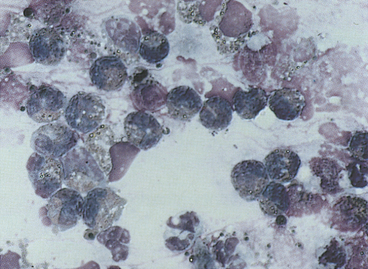
Many different types of cells (e.g., ciliated and nonciliated columnar cells, ciliated and nonciliated cuboidal cells, alveolar macrophages, neutrophils, eosinophils, lymphocytes, mast cells, erythrocytes, and dysplastic and neoplastic cells) may be seen with TW/BAL (see Tables 16-1 and 16-2). Ciliated and nonciliated columnar and cuboidal cells and alveolar macrophages are the cell types seen in washings from normal dogs and cats. They are also seen in many disease states unless the washed area is filled with exudative secretions or the disease process has obliterated normal lung parenchyma.
Columnar and Cuboidal Cells
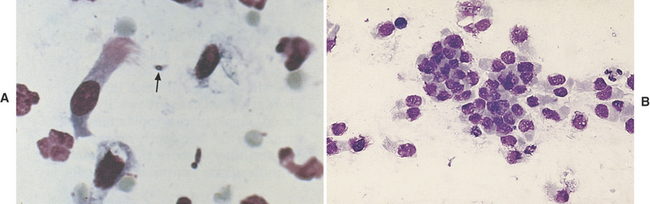
Ciliated columnar cells (Figures 16-4 and 16-5) have an elongated or cone shape, with cilia on their flattened apical ends. The nucleus, which is generally round to oval with a finely granular chromatin pattern, is present in the basal end of the cells, which often terminate in a thin tail.4 The ciliated cuboidal cells look similar to the ciliated columnar cells except that the cuboidal cells are as wide as they are tall. Nonciliated columnar and cuboidal cells look identical to their ciliated counterparts, except for the absence of cilia. These cell types are normal findings in TW/BAL. If these cell types are predominant in a sample, the washing procedure probably sampled mainly bronchi and bronchioles (as opposed to alveolar spaces).
Cuboidal and columnar epithelial cells may be present individually or in clusters (see Figure 16-4, B). Depending on the orientation of the cell on the slide (especially with cells in clusters), the cuboidal/columnar nature of the cells and cilia may be difficult to visualize. This is of little clinical significance, but these cells must not be interpreted as abnormal cell types.4 Also, the majority of the columnar cells may be poorly preserved in many washes (see Figure 16-4, B) as a result of the low protein fluid in which they are collected. Cells traumatized during slide preparation may show irregular nuclear outlines or be overtly ruptured (e.g., smudge cells).
Goblet Cells
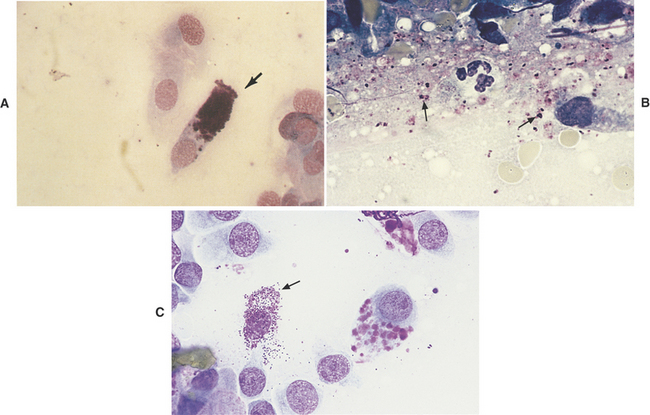
Goblet cells (see Figure 16-5) are mucus-producing bronchial cells that are generally elongated (i.e., columnar) with a basally placed nucleus and round granules of mucin, which frequently distend the cytoplasm.4 Occasionally, the cytoplasm is so distended that the cell appears round. The granules stain from red to blue to clear with Romanowsky (e.g., Giemsa-Wright) stains. Free granules from ruptured goblet cells may be seen in the smear (see Figure 16-5, B). The shape of the cells and the large size of the granules are helpful in differentiating these cells from mast cells (see Figure 16-5, C). Goblet cells are not commonly seen; however, any chronic pulmonary irritant may result in increased numbers of goblet cells.
Macrophages
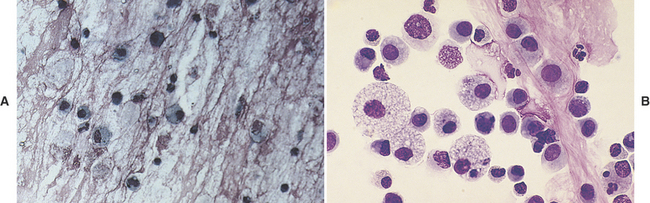
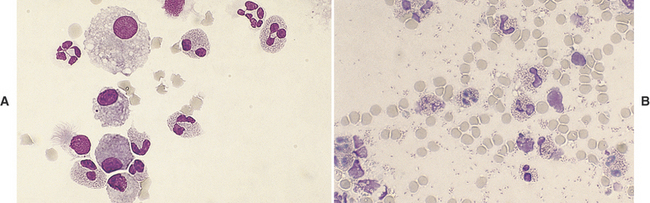
Alveolar macrophages (Figure 16-6) (see also Figures 16-8, 16-12, and 16-17) are readily found and are often the predominant cell type in TW/BAL from clinically normal animals. They are present in samples that have adequately washed the alveolar spaces and therefore are a useful indicator of sample adequacy. The nucleus is round to bean-shaped and eccentrically positioned. A binucleate alveolar macrophage is rarely seen in clinically normal animals. Alveolar macrophages have abundant blue-gray granular cytoplasm. When they become activated, their cytoplasm becomes more abundant and vacuolated (i.e., foamy) and may contain phagocytized material (see Figure 16-6, B).29
Neutrophils
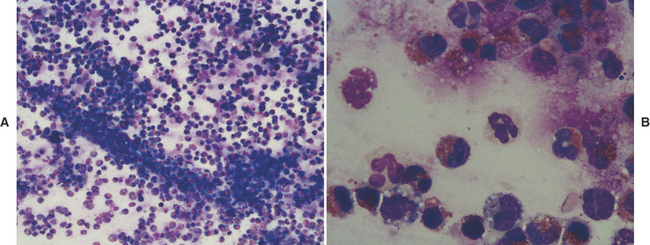
TW/BAL neutrophils look like peripheral blood neutrophils (see Figures 16-10 and 16-11), although degenerative changes may be present. Increased numbers of neutrophils indicate inflammation; see the discussion on inflammation later in this chapter.
Eosinophils
Eosinophils (Figures 16-7 to 16-9) are polymorphonuclear granulocytes that contain intracytoplasmic granules, many of which have an affinity for the acid dye, eosin (i.e., eosinophilic), which stains them red with Romanowsky stains.36 Increased numbers of eosinophils indicate a hypersensitivity reaction that is either allergic or parasitic5; see discussion on hypersensitivity later in this chapter.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree