Nicholas J. Masters, Edmund Flach
Tragulidae, Moschidae, and Cervidae
Deer have been known to, and hunted by, human beings for millennia, and the annual cycle of antler growth has long been a source of fascination and folklore. Currently, veterinarians may work with deer and their relatives in zoos, in farms, and in the wild, with an increasing involvement in conservation programs for endangered deer species (both in situ and ex situ). Many names have been given to age or sex categories of deer of different species, but to avoid confusion, the authors refer to adult males as stags, adult females as hinds, and juveniles as calves. This chapter focuses on seasonal species kept in zoos in Europe and North America, but a useful reference to neotropical species has recently been published.15
General Biology
The taxonomy of the chevrotains—or mouse deer (Family Tragulidae), musk deer (Moschidae), and true deer (Cervidae)—has changed over recent years, but in the recent revision by Groves and Grubb,26 all have been classified as members of the suborder Ruminantia within the order Artiodactyla. The 10 species in the three genera of Tragulidae are sufficiently distinct to have a separate infraorder (Tragulina) from all other ruminants (Percora). The general consensus is that seven Moschus species (Moschidae) exist, but numbers of species and genera within Cervidae vary among texts.26,27,42,57,72 The authors have followed the classification of Wilson and Mittermeier (2010)72 and have placed 53 species in 18 genera, with Hydropotes included in the suborder Capriolinae (Table 62-1).
TABLE 62-1
Basic Biologic Information and Conservation Status of Tragulidae, Moschidae, and Cervidae72
| Scientific Name (number of species) | Common Name(s) | Weight Range (kg) | Distribution | Species and Subspecies of Conservation Concern |
| TRAGULIDAE | ||||
| Hyemoschus (1) | Water chevrotain | 7–16 | C and W Africa | — |
| Moschiola (3) | Chevrotain | 2.4–3 | SC and SE Asia | — |
| Tragulus (6) | Mouse deer | 1.5–4.5 | SC and SE Asia | Balabac mouse deer (T. nigricans) EN |
| MOSCHIDAE | ||||
| Moschus (7) | Musk deer | 6–17 | Asia | All EN (6) or VU (1) |
| CERVIDAE | ||||
| Capreolinae | ||||
| Alces (1) | Moose (Eur. elk) | 280–600 | Circumpolar | — |
| Blastocerus (1) | Marsh deer | 70–130 | S. America | VU |
| Capreolus (2) | Roe deer | 17–50 | Eurasia | |
| Hippocamelus (2) | Taruka, huemel | 45–75 | S. America | S Andean huemel (H. bisulcus) EN N Andean huemel (H. antisensis) VU |
| Hydropotes (1) | Chinese water deer | 11–15 | SE Asia | VU |
| Mazama (10) | Brocket | 8–35 | C and S America | Five species VU |
| Odocoileus (2) | Mule and white-tailed deer | 25–130 | Americas | — |
| Ozotoceros (1) | Pampas deer | 22–40 | S America | — |
| Pudu (2) | Pudu | 5–14 | S America | Both VU |
| Rangifer (1) | Reindeer (caribou) | 55–170 | Circumpolar | — |
| Cervinae | ||||
| Axis (4) | Axis deer (chital), hog deer | 30–110 | SE & S Asia | Bawean deer (A. kuhlii) CR Calamian deer (A. calamianensis) EN Hog deer (A. porcinus) VU |
| Cervus (5) | Red deer (Am. Elk), sika deer | 20–400 | Eurasia, N Africa, America | White-lipped deer (C. albirostrus) VU |
| Dama (2) | Fallow deer | 35–140 | Asia | Persian fallow (D. mesopotomica) EN |
| Elaphodus (1) | Tufted deer | 17–30 | S Asia | — |
| Elaphurus (1) | Père David deer | 140–220 | E Asia | EW |
| Muntiacus (11) | Muntjac | 12–35 | SE and S Asia | Giant muntjac (M. vuquangensis) EN Black muntjac (M. crinifrons) VU |
| Rucervus (2) | Barasingha, brow-antlered (Eld’s) deer | 60–200 | S Asia | Brow-antlered (R. eldii) EN Barasingha (R. duvaucelii) VU |
| Rusa (4) | Javan (rusa) and sambar deer | 40–350 | SE and S Asia | Phillipine spotted (R. alfredi) EN Others VU |
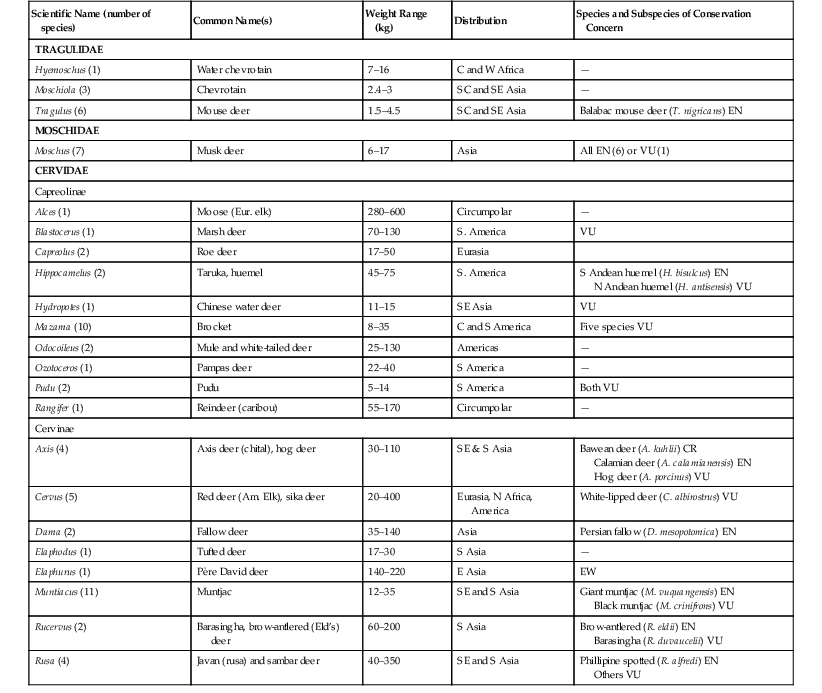
C, Central; CR, critically endangered; E, east; EN, endangered; EW, extinct in the wild; N, north; S, south; VU, vulnerable; W, west.
All deer and their relatives are herbivorous, but they may be browsers, grazers, or intermediate feeders (see Feeding). Body mass tends to be greater in males, although exceptions (e.g., some Tragulidae) do exist. Social structure varies from solitary existence to herd living. The group, as a whole, is extremely widespread, from the arctic tundra to tropical forests, and deer are arguably the most successful ungulates. Despite this, many species and subspecies are declining in population because of exploitation by humans, habitat loss, and environmental threats (see Table 62-1). One species, the Père David deer (Elaphurus davidianus), has become extinct in the wild, but the species has been reintroduced in China, thanks to long-term captive programs.
Unique Anatomy
The chevrotains (Tragulidae) have many ruminant features such as a four-chambered stomach (with a poorly developed omasum), no upper incisor teeth, and incisor-like lower canines, but they also resemble pigs and hippopotami in having four toes with supporting bones, as well as incomplete fusion of the third and fourth metacarpals and metatarsals.42,57 Members of this family lack antlers and have elongated upper canines as in musk deer and Chinese water deer (Hydropotes inermis) and, like musk deer, also possess a gallbladder and lack preorbital scent glands. However, they do possess a chin gland.
Musk deer (Moschidae) lack antlers, have a gallbladder, and possess just one pair of mammary glands. They have no facial glands but have caudal glands ventral to the tail and well-developed preputial or musk glands, from which the commercially valuable musk is obtained. Moschidae used to be included as a subfamily in Cervidae but has now been raised to family status.26,72
The defining characteristic of the true deer (Cervidae) is the possession of antlers (Figure 62-1). Males of all species, except the Chinese water deer, grow antlers each year before the breeding season and lose them later. In the species that comprises the reindeer, or caribou (Rangifer tarandus), both sexes have antlers. The size and complexity of antlers increases with distance from the equator, as climatic and nutritional conditions become more exacting. Hinds select males that can thrive best in the environment, and the choice of a female may be the most important factor for the evolution of large antlers. Antler growth occurs annually under hormonal control. The antlers arise from the frontal bones and skin and, until fully grown, they are covered with a highly vascularized and sensitive skin known as velvet. As the mating season approaches, the testosterone level rises, the antlers harden, and the velvet dries. Stags may rub their antlers against objects to remove the flaking velvet. After the mating season, the testosterone level declines, and a layer of bone-dissolving cells invades the base of the antlers, causing them to fall off. Each year, the size and, in some species, the complexity of antlers increase until full maturity. Antler growth has a less well-defined seasonality in tropical and subtropical zones.

Deer possess a range of specialized scent glands, most commonly preorbital in location but also occurring on the limbs. Deer have no gallbladder, and female deer have two pairs of inguinal mammary glands. The skeleton has evolved for running and jumping, with reductions of the ulna and the fibula, loss of the first digit, reductions of digits II and V, and fusion of the third and fourth metacarpals and metatarsals to form cannon bones. The location of the vestigial metacarpal bones II and V (splint bones) is the basis for differentiation of the subfamily Cervinae (proximal or plesiometacarpalian) from the Capreolinae (distal or telemetacarpalian). The Chinese water deer has been placed in the subfamily Capreolinae, as it is telemetacarpalian and is phylogenetically very close to the roe deer (Capreolus capreolus); this is based on mitochondrial cytochrome analysis,60 which suggests that the loss of antlers is a secondary characteristic.
Special Housing Requirements
The majority of deer species may be kept in natural-ground paddocks bounded by fences or walls that may have to be 2.5 to 3 meters (m) high for the larger species and well buried. High-tensile steel netting 1.8 to 2 m high, with posts 5 to 8 m apart, commonly is used for farming red deer (Cervus elaphus) and fallow deer (Dama dama), but chain-link fencing and electric fencing also may be used. With any form of fencing comes the risk that stags may get their antlers caught. Cattle grids may be used to stop deer exiting enclosures via roadways, but they should be at least 2 m (preferably 3 m) wide. Shelters should be sufficient for the number of animals, and some should be partially enclosed. Trees and shrubs help provide windbreaks and cover but must be protected from bark-stripping and extensive browsing. Dead trees and logs act as rubbing posts when stags are losing their velvet.
Small, tropical species cannot be kept outdoors all year in a temperate climate and require heated housing. Many zoos keep chevrotains in nocturnal houses with reversed lighting.
Feeding
All chevrotains and deer (musk and true) are herbivorous, but they range from species that inhabit forests and bush, for example, musk, muntjac (Muntiacus species), brocket (Mazama species), roe deer, and moose (Alces alces), which are browsers on dicotyledonous plant material (leaves and twigs of trees and shrubs, as well as herbs and forbs), to grazers such as fallow deer and Père David deer, which feed on monocotyledonous plant material (primarily the leaves or blades of grass). The majority of species are intermediate or mixed opportunistic feeders. The feeding habits are reflected in gastrointestinal (GI) anatomy and physiology; thus, the greater the proportion of grass and roughage in the natural diet (the grazers), the greater is the retention time of fiber in the rumen and the less frequently the animal needs to feed.31 Grazing species also have hypsodont dentition (high-crowned teeth) adapted to abrasive wear, whereas browsers have brachyodont (low-crowned) dentition for attritional wear.35 Browsers may produce tannin-binding and similar proteins in their saliva to neutralize the secondary compounds present in woody plants.12 Unfortunately, the feeding of browsers in captivity has traditionally been problematic, with many species avoiding grass and grass-based forage and therefore ingesting higher proportions of concentrate pellets. This is suspected to have led to cases of chronic ruminal acidosis and ill-thrift, for example, “wasting syndrome complex” in moose,12 and a decreased relative life-expectancy in captivity in relation to grazing species.53 Ruminal acidosis may also be seen in wild deer that ingest large quantities of supplementary food.63 Deer in seasonal climates, especially browsers, build up body fat during spring and summer (or wet season) to provide energy for the rut and winter (or dry season) when the quantity and nutrient quality of plants is low.
Feeding in captivity is based on management goals. For deer farmers, supplementing pasture grazing so that animals meet target weights is important, as is keeping the breeding stags and hinds in good condition for rutting or mating, pregnancy, and lactation. In zoos and deer parks, slower growth is acceptable, or even preferred, but supplementary feeding may be necessary during the winter or dry season and also for pregnant and lactating hinds and their calves. In the temperate areas of the northern hemisphere, grass paddocks and an appropriate stocking density provide energy and protein for grazing species in spring and summer, with hay offered during the fall and winter seasons and low-protein concentrates (maximum 12%) only offered if the hay is of poor quality. Indeed, excess feeding of pregnant Père David hinds may result in dystocia caused by oversized calves. Intermediate or mixed deer species require hay during the fall and winter seasons, plus additional concentrates (maximum 14% crude protein). Root crops have been used successfully for winter feeding of farmed deer. Browsers should receive a browser-specific concentrate diet (high-fiber and low-energy diet, preferably with beet pulp as a pectin source to avoid grains) plus browse (fresh, dried, or ensiled), with or without alfalfa (lucerne).12 Easily digested energy sources such as starch are not necessary and should be avoided.47 If the natural pattern of reduced appetite during the winter season is followed, deer may lose up to 10% of body weight because of higher energy demands (thermoregulation, activity). This weight is regained in the spring season, with juveniles especially showing a compensatory growth spurt. Deer overwintered indoors will have much lower energy requirements, so supplementary feeding may be greatly reduced.
Nutrient requirements, including minerals and vitamins, for selected deer have been published,56 but in general, commercial cattle salt or mineral blocks are suitable, with the addition of specific minerals and vitamins if local grazing or the forage fed are deficient.
Restraint and Handling20,22,29,68
Behavioral Restraint
Captive artiodactyls, including deer, can be habituated to routine management practices and, with appropriate handling facilities and expertise (see below), it is possible to undertake simple veterinary procedures, including venipuncture, vaccination, and physical examination within restraint devices.
Physical Restraint
The correct use of appropriate physical restraint may be the safest and most efficient way to handle wild, zoo, and game-farmed deer for management, treatment, diagnostic, and research purposes, but good planning and teamwork are essential to prevent capture stress and subsequent myopathy.
Small deer may be confined in padded boxes, entrapped with the use of net-guns, or driven into collapsible nets for manual capture. However, they should only be restrained in lateral recumbency for short periods of 15 minutes or less; any longer procedure would require sedation or anesthesia. Hobbles may be applied to the ipsilateral forelimbs and hindlimbs at the level of the metacarpophalangeal joint for safety, and the use of blindfolds may calm the animal.
Sophisticated handling systems have been designed to be used in farmed species to allow deer to be moved, separated, loaded for transport, and restrained for management and veterinary procedures.28 Typically, collecting funnels from paddocks lead to chutes and yards with smooth, solid walls (e.g., plywood attached to metal frames), sufficiently high to prevent animals from jumping out and with viewing holes so that deer and handlers are able to see and be aware of each other. Wild deer may be encouraged into the funnels by regular feeding or by driving (e.g., by helicopter). Once inside, deer may be separated and channeled into different raceways from sorting pens that are covered to be kept dark, especially when dealing with nervous species. Particular consideration should be paid to the specific flight distances of the species to be handled. Deer held in crushes (manual or hydraulic) with a drop floor may be examined and many minimally invasive procedures carried out without causing undue stress. Male cervids and both sexes of reindeer should not be restrained when antlers are in growth because of the potential for major blood loss and severe pain associated with trauma. Once hardened, the antlers should be removed if the stags (or reindeer hinds) are to go through a handling system.
Chemical: Anesthesia
Deer are prone to capture myopathy, hyperthermia and trauma, so any anesthetic procedure should be carefully planned, taking into account the method of capture, ambient temperature, physiology of species, and clinical condition of the individual. Table 62-2 lists dose rates of commonly used chemical restraint agents, some for specific species. The data are taken from a variety of sources, from different conditions, which explains the wide ranges shown. Captive deer often have drug requirements very different from those of free-ranging animals, so this must be taken into consideration.11 Ideally, therefore, advice should be sought from those regularly immobilizing the species of interest, and doses should be tailored to each circumstance.
TABLE 62-2
Chemical Restraint Agents Used for Tragulidae, Moschidae, and Cervidae
| Agent | Species | Dosage* | Reversal Agent and Dosage |
| Diazepam | All | 0.5–2.0 milligram per kilogram (mg/kg), IV or oral18 | Flumazenil 0.04–0.15 mg/kg |
| Xylazine | All White-tailed deer and mule deer Wapiti/North American elk (Cervus canadensis) Red deer | 0.4–8.0 mg/kg, IM ( dose IV)18 dose IV)182.0–3.0 mg/kg, IM10 1.0 mg/kg, IM10 0.5–1.0 mg/kg, IM11 | Yohimbine 0.10–0.20 mg/kg (half IM/half IV), or Atipamezole 0.04–0.8 mg/kg, IV (i.e., 0.1 mg/mg xylazine) |
| Etorphine/ acepromazine | All | 0.02–0.05 mg/kg; and 0.08–0.2 mg/kg, IM18 | Diprenorphine 0.027–0.066 mg/kg, IV |
| Etorphine/ acepromazine/ xylazine | All | 0.01–0.06 mg/kg; 0.04–0.24 mg/kg; and 0.25–0.60 mg/kg, IM18 | Diprenorphine for etorphine, 0.013–0.08 mg/kg, IV Yohimbine or atipamezole for xylazine |
| Carfentanil | All | 0.006–0.03 mg/kg, IM18 | Diprenorphine 0.06–0.3 mg/kg, or naltrexone 0.06–0.3 mg/kg |
| Carfentanil/ xylazine | Wapiti/North American elk | 0.01 mg/kg; and 0.1 mg/kg, IM52 | Naltrexone for carfentanil |
| Thiafentanil | White-tailed deer Moose | 3 mg total dose39 10 mg total dose39 | Naltrexone 10 mg/mg thiafentanil, IM |
| Thiafentanil/ xylazine | Wapiti/North American elk Mule deer | 12–15 mg thiafentanil, and 50 mg xylazine, IM39 10–12 mg thiafentanil; and 100 mg xylazine, IM; or 0.15–0.20 mg/kg thiafentanil and 100 mg xylazine, IM39 | Naltrexone for thiafentanil |
| Xylazine/ ketamine | All White-tailed deer and mule deer Wapiti/North American elk | 0.5–23 mg/kg and 2.7–18.7 mg/kg IM18 2.0 mg/kg, and 3–4 mg/kg IM10 1.0 mg/kg, and 3–4 mg/kg IM10 | Yohimbine or atipamezole for xylazine |
| Medetomidine/ ketamine | All Red deer Moose Reindeer/caribou | 0.05–0.10 mg/kg and 0.8–3.2 mg/kg, IM18 0.11 mg/kg; and 2.2 mg/kg, IM3 0.06 mg/kg, and 1.5 mg/kg IM4 0.1 mg/kg, and 2.5 mg/kg IM2 | Atipamezole 0.25–0.5 mg/kg, split IV and IM (i.e., 5 mg/mg medetomidine) |
| Tiletamine/ zolazepam | All | 2.9–20 mg/kg, IM (33 mg/kg, IM, for fallow)18 | Flumazenil, 0.11–0.77 mg/kg |
| Tiletamine/ zolazepam/ xylazine | White-tailed deer and mule deer Moose | 4.4 mg/kg; and 2.2 mg/kg, IM54 3.0 mg/kg; and 1.5 mg/kg, IM11 | Yohimbine or atipamezole for xylazine |
| Tiletamine/ zolazepam/ medetomidine | All Fallow deer | 0.7–1.3 mg/kg, and 0.08–0.12 mg/kg IM18 1.0 mg/kg, and 0.1 mg/kg IM17 | Flumazenil 0.03–0.1 mg/kg Atipamezole 0.4–0.6 mg/kg, split IV and IM |
| Butorphanol/ azaperone/ medetomidine (BAM) | White-tailed deer and mule deer Wapiti/North American elk | 0.58 mg/kg; and 0.37 mg/kg; and 0.19 mg/kg, IM9 0.11 mg/kg; and 0.07 mg/kg; and 0.05 mg/kg, IM9 | Atipamezole 3.0 mg/mg medetomidine; and naltrexone 50 mg/ butorphanol 30 mg, IM |
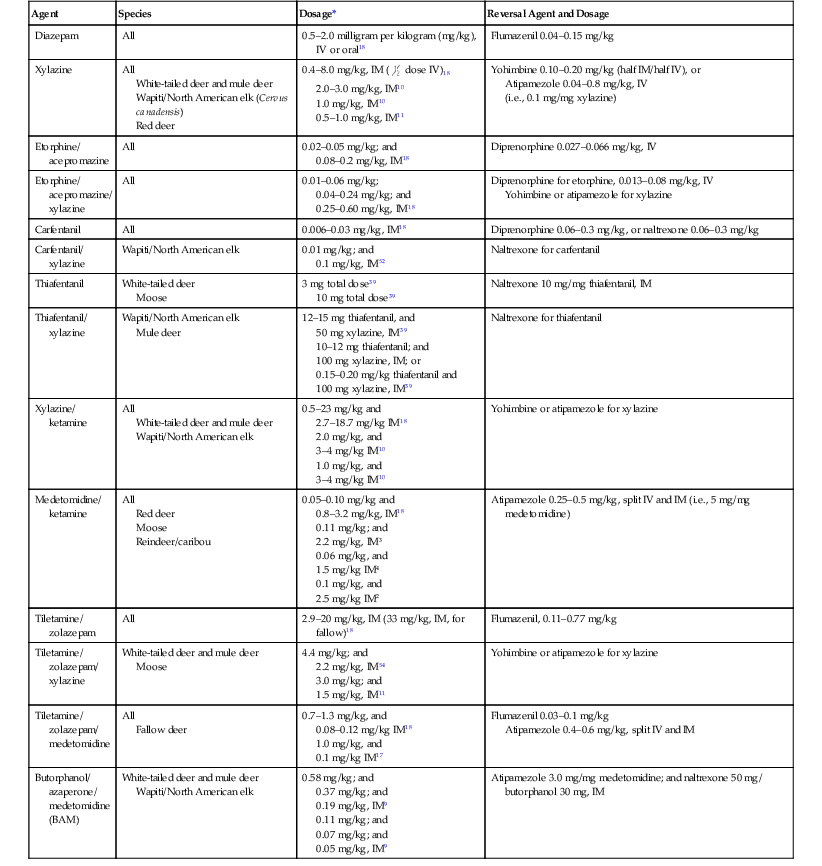
* IV, Intravenously; IM, intramuscularly.
Sedation alone may be unrewarding in deer, except in tamer animals such as reindeer, when an α2-agonist such as xylazine may be injected intravenously (IV). Relatively high doses of xylazine may be administered intramuscularly (IM) to produce recumbent sedation. Excited animals may overcome the drug’s sedative effects, so stimulation must be minimized during induction; the animal should be left alone until recumbent and then approached cautiously and quietly to be blindfolded. For longer or painful procedures, an intravenous bolus of ketamine (1 milligram per kilogram [mg/kg]) may be given, with additional intravenous boluses (1–2 mg/kg) as required. However, intubation and inhalation anesthesia are preferred.11 Diazepam may be given orally (PO) before a stressful situation, but the effect of this drug is variable.
Xylazine and ketamine are still a useful combination for the immobilization of small deer. For example, an adult Chinese water deer requires approximately 1.5 milliliters (mL) of the “Hellabrun” mixture (125 mg/mL xylazine plus 100 mg/mL ketamine). Darting medium- and large-sized deer with an opioid agent is more economical and practical; etorphine hydrochloride (HCl), carfentanil citrate, or, most recently, thiafentanil oxalate, is used usually in combination with an α2-agonist to reduce excitation, muscle tremors, and respiratory depression.74 The choice of opioid primarily depends on availability, but all opioids can be antagonized. However, re-narcotization may occur some hours after reversal because of the shorter duration of action of some antagonists compared with the opioid drug. Animals should be monitored for 72 hours with administration of further doses of the antagonist, if necessary. Compared with the other opioids, thiafentanil oxalate has the advantages of more rapid induction (reducing chase time), equal or greater potency, and a shorter half-life, thus reducing the likelihood of re-narcotization.39 The combination of medetomidine and ketamine is increasingly preferred because of the inherent risks in the use of opioids, and the lower required dose of ketamine compared with the xylazine–ketamine mixture.33 The tiletamine–zolazepam combination results in a long recovery period, unless the zolazepam is reversed with flumazenil. Alternatively, as flumazenil is expensive, a lower dose of tiletamine–zolazepam may be used with xylazine or medetomidine, and recovery, after reversal of the α2-agonist, is much quicker. This combination is reported to be ideal for the immobilization of fallow deer.17 Since 2008, the combination of butorphanol, azaperone, and medetomidine (BAM) has been frequently used in hoofstock. Hyperthermia is avoided, and excellent respiration and good muscle relaxation are achieved. Reversal following injections of atipamezole (3 mg/1 mg medetomidine) and naltrexone (50 mg/30 mg butorphanol) is usually complete within 10 minutes. Over 1000 white-tailed deer (Odocoileus virginianus) have been anesthetized successfully with BAM, but results in fallow deer have been disappointing.9
After induction, deer should be positioned in sternal recumbency, with the neck extended but the nose pointing slightly toward the ground, to maintain a patent airway and to reduce the likelihood of aspiration of any regurgitated ruminal contents and development of ruminal tympany. Ideally, deer should be intubated (see below) and given supplemental oxygen; if not, oxygen may be provided via nasal insufflation (to the level of the medial canthus of the eye). In either case, oxygen flow is adjusted to maintain saturation of peripheral oxygen (SpO2) of 95% or greater. Intubation may be difficult in deer because of the depth of the larynx, the narrowness of the buccal cavity, and the long and mobile epiglottis but is facilitated by correct positioning, the use of a long laryngoscope, a stylet to stiffen the endotracheal tube, and increasing anesthetic depth with an intravenous bolus of ketamine (1–2 mg/kg) or propofol (2–4 mg/kg).
For elective inhalation anesthesia, food should be withheld for 24 to 36 hours and water for 12 hours prior to induction. The principles of anesthesia and monitoring are the same as for domestic ruminants. At the end of all procedures during which the animal was intubated, extubation, with the cuff still partially inflated, should only be performed once the swallowing reflex has returned. When reversing α2-agonists, the appropriate agents should be divided and half-doses given IM and IV.10
Capture myopathy is a potentially life-threatening complication of anesthesia that may present in acute, subacute, or chronic forms. Treatment is based around the correction of shock and acid–base balance, the maintenance of normothermia, and oxygenation. Results are generally unrewarding, so prevention is better and is achieved by ensuring that the animals are not deficient in vitamin E and selenium and by minimizing the duration of chase and restraint.11,59
Chemical: Neuroleptics
Two classes of human antipsychotic drugs (butyrophenones and phenothiazines) have been used as tranquilizers in wild animals since the 1980s for loading, transportation, and acclimatization. These drugs reduce dopamine neurotransmission, which minimizes stress and reduces the risk of trauma and capture myopathy. However, extrapyramidal side effects may occur. The butyrophenones azaperone and haloperidol, injected IM or IV, are shorter-acting (up to 72 hours), and azaperone (0.2 mg/kg) may be given immediately after anesthetic reversal for short translocations (<6 hours). The phenothiazines are slower in onset but longer-acting and may only be given IM.25 Zuclopenthixol acetate (1 mg/kg) has been shown to reduce flight distance and stress and increase water and food consumption.61 Zuclopenthixol acetate lasts up to 4 days, perphenazine enanthate up to 10 days, and pipothiazine palmitate up to 21 days, with their time to onset of action correspondingly long. Therefore, they are often used in combination.
Surgery
Common procedures that require anesthesia are the amputation of antlers, investigation of lameness or preventive hoof work, treatment of dystocia, and, if necessary, cesarean section. These and other surgical procedures are the same as performed in domestic ruminants. Local anesthesia may be useful to produce “ring block” and effective analgesia for the removal of antlers in velvet (either complete, or partial following traumatic injury), and this is preferable to antler pedicle compression.34
Physical Examination and Diagnostics
The diagnostic process for investigating disease in chevrotains, musk deer, and true deer is no different from that in other ruminants but takes into account the unique anatomic and physiologic features mentioned earlier. Blood samples usually are collected from the jugular vein or the ventral coccygeal vein. Tables 62-3 and 62-4 list selected hematologic and biochemical parameters. Interpretation of results should take into consideration the method of capture,46 handling, time delays after collection,41 and also differences between sexes and age categories.64
TABLE 62-3
Reference Ranges for Hematologic Parameters of Selected Tragulidae, Moschidae, and Cervidae
| Parameter | Tragulus napu | Moschus moschiferus* | Odocoileus virginianus | Pudu puda | Rangifer tarandus | Axis axis | Cervus elaphus | Muntiacus reevesi |
| Red blood cell count (×106/microliter (µL)) | 61.9–168.3 | 10–12 | 8.2–15.8 | 7.8–11.0 | 8.8–11.6 | 7.4–15.7 | 6.6–10.6 | 8.6–21.9 |
| Hemoglobin, gram per deciliter (g/dL) | 11.0–15.6 | 14–18 | 11.5–16.7 | 12.6–17.6 | 13.5–20.1 | 10.6–19.4 | 10.8–18.2 | 11.7–17.7 |
| Hematocrit (%) | 37.6–59.6 | 39–51 | 31.2–49.4 | 35.4–51.8 | 38.8–55.2 | 31.4–57.0 | 31.7–50.1 | 33.1–50.5 |
| Mean corpuscular volume (fL) | 2.5†–46.3 | 36–42 | 24.1–47.3 | 41.2–53.0 | 42.4–52.6 | 23.4–57.4 | 40.4–58.4 | 12.2–54.8 |
| Mean corpuscular hemoglobin, picogram (pg) | 0.8†–14.3 | 13–15 | 8.3–16.3 | 14.0–19.0 | 15.3–18.7 | 8.0–20.4 | 14.0–20.6 | 4.6–19.2 |
| Mean corpuscular hemoglobin concentration (g/dL) | 21.8–32.6 | 33–39 | 32.8–37.8 | 30.3–38.3 | 31.8–38.4 | 27.2–40.4 | 31.0–38.0 | 31.2–40.0 |
| White blood cell count (×103/µL) | 5.32–16.36 | 1–5 | 1.82–5.26 | 4.91–10.19 | 2.41–8.51 | 2.16–6.40 | 2.43–6.85 | 2.63–8.13 |
| Segmented neutrophils (×103/µL) | 1.73–7.37 | 0.2–1.8 | 0.50–3.58 | 1.43–4.66 | 0.68–5.42 | 0.37–4.54 | 0.97–4.51 | 1.07–5.40 |
| Lymphocytes (×103/µL) | 2.29–7.79 | 0.5–2.5 | 0.54–1.91 | 2.08–5.70 | 0.55–3.22 | 0.62–2.40 | 0.50–2.35 | 0.63–3.23 |
| Monocytes (×103/µL) | 0.05†–1.20 | 0.1–0.3 | 0.01†–0.26 | 0.09–0.63 | 0.00–0.43 | 0.00†–0.32 | 0.02–0.35 | 0.00†–0.40 |
| Eosinophils (×103/µL) | 0.09–0.92 | 0.0–0.1 | 0.0–0.54 | 0.06–0.68 | 0.03–0.78 | 0.00†–0.29 | 0.00†–0.88 | 0.00†–0.27 |
| Basophils (×103/µL) | 0.00†–0.44 | 0.0–0.1 | 0.01–0.08 | 0.04–0.16 | 0.00–0.23 | 0.01–0.06 | 0.00†–0.21 | 0.01–0.11 |
| Platelets (×103/µL) | No data | 23–277 | 287–583 | 115–435 | 275–521 | 26–570 | 83–509 | 160–422 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


