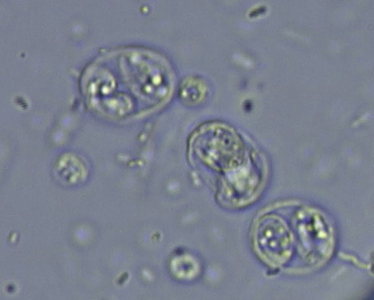
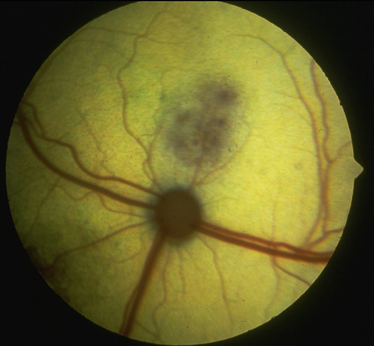
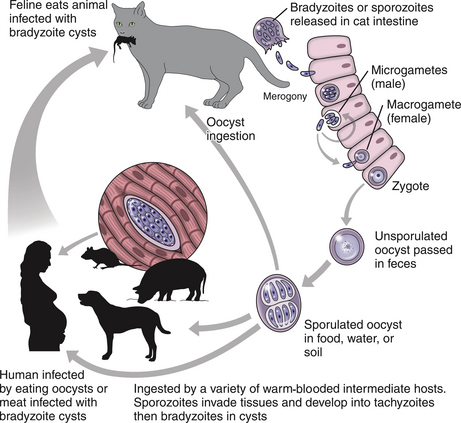
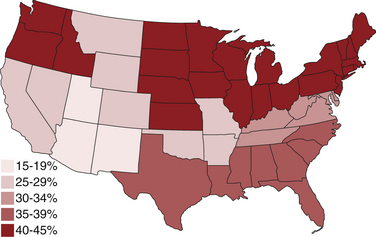
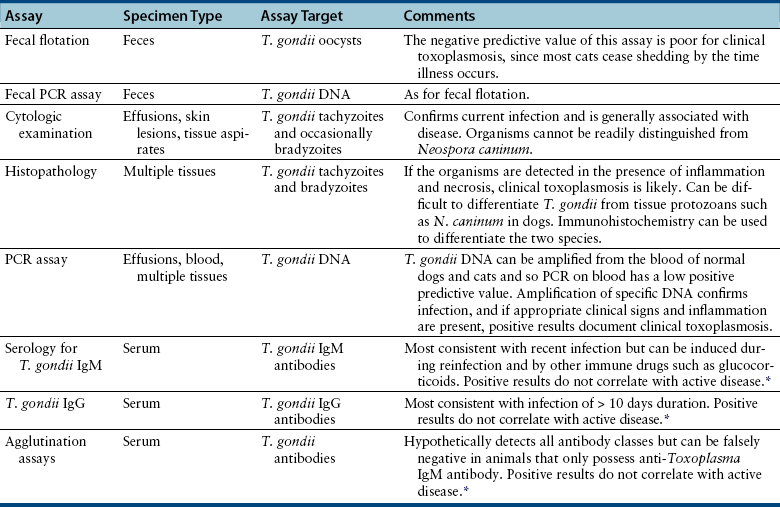
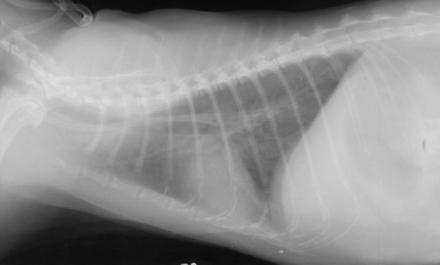
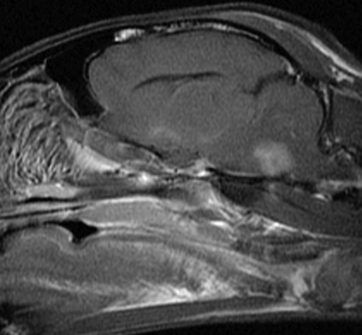
Chapter 72 Toxoplasma gondii is a coccidian that is one of the most prevalent parasites infecting warm-blooded vertebrates around the world.2–4 Only cats complete the sexual phase in the gastrointestinal tract (also known as the enteroepithelial cycle) and pass environmentally resistant oocysts in feces (Figure 72-1). Sporozoites develop within shed oocysts after 1 to 5 days of exposure to oxygen and appropriate environmental temperature and humidity (Figure 72-2). This process is known as sporulation. After oocyst ingestion, released sporozoites can penetrate the intestinal tracts of cats or intermediate hosts and disseminate in blood or lymph as tachyzoites during active infection. T. gondii can penetrate most mammalian cells and replicates asexually within infected cells until the cell is destroyed. If an appropriate immune response occurs, replication of tachyzoites is attenuated and slowly dividing bradyzoites develop that persist within cysts in extraintestinal tissues. These tissue cysts form readily in the central nervous system (CNS), muscles, and visceral organs. Live bradyzoites may persist in tissue cysts for the life of the host. FIGURE 72-1 Life cycle of Toxoplasma gondii. Cats are most often infected when they ingest bradyzoite cysts in tissues of prey. The bradyzoites transform into merozoites and undergo repeated cycles of schizogony in the cat’s intestinal tract. The merozoites then transform into microgametes (male) and macrogametes (female). When a microgamete penetrates a macrogamete, a zygote forms. The zygote is shed as an unsporulated oocyst in the feces, which is not infectious. Sporulation occurs after 1 to 5 days. The extraintestinal cycle occurs in cats or other intermediate hosts after they ingest sporulated oocysts or tissue cysts. Sporozoites penetrate intestinal cells and transform into tachyzoites, which rapidly multiply in nucleated cells throughout the body in the face of immunosuppression. This can result in severe disease in the developing fetus. Ultimately organisms are contained by the immune system within bradyzoite cysts in muscle, brain, and visceral organs (latent infection). It is likely that most T. gondii–infected cats and dogs harbor tissue cysts for life. Thus, the presence of serum antibodies is likely to indicate current infection. T. gondii seroprevalence rates vary by the lifestyle of the cat or dog. In general, increased seroprevalence correlates with increased age as a result of increased risk of exposure over time. It also correlates with outdoor exposure, since animals with access to the outdoors are most likely to contact infected intermediate hosts. In a recent study of clinically ill cats, T. gondii antibodies were detected in 31.6% of the 12,628 cats tested.5 The seroprevalence was lowest in regions with low humidity (Figure 72-3). In another study of feral cats in Florida, T. gondii antibodies were detected in 12.1% of the cats.6 Although T. gondii infection is common among cats, the oocyst shedding period is generally less than 21 days. Thus, detection of T. gondii oocysts in feline feces is uncommon. For example, in two studies in the United States, T. gondii oocysts were detected in feces of fewer than 1% of cats.7,8 FIGURE 72-3 Continental United States regional seroprevalence in clinically ill cats. Results were based on the presence of Toxoplasma gondii-specific IgG or IgM in serum. Information was available from 3,640 serum specimens. (From Vollaire MR, Radecki SV, Lappin MR. Seroprevalence of Toxoplasma gondii antibodies in clinically ill cats in the United States. Am J Vet Res 2005;66:874-877.) Dogs do not produce T. gondii oocysts, but they can mechanically transmit oocysts after they ingest feline feces.9 The tissue phases of T. gondii infection occur in dogs and may be associated with clinical disease. Approximately 20% of dogs in the United States are seropositive for T. gondii antibodies.10 Before 1988, many dogs diagnosed with toxoplasmosis based on histologic evaluation were in fact infected with Neospora caninum (see Chapter 73). Infection of warm-blooded vertebrates occurs after ingestion of any of the three life stages of T. gondii (sporozoite, tachyzoites, bradyzoites) or it can occur by transplacental transmission. Cats can also be infected through the transmammary route.11 In dogs, evidence for venereal transmission also exists, and repeated transplacental infection has been documented.12,13 Most cats are not coprophagic, so most are infected when they ingest T. gondii bradyzoites in tissue cysts during carnivorous feeding. After ingestion, the bradyzoites transform into merozoites, which replicate in the epithelial cells of the intestinal tract, then transform into microgametes and macrogametes. When a microgamete penetrates a macrogamete, a zygote forms. The zygote then transforms into an unsporulated oocyst, which is shed in feces for 3 to 21 days. After sporulation (formation of sporozoites within the oocyst), oocysts can survive in the environment for months to years and are resistant to most disinfectants. The T. gondii oocyst shedding prepatent period is stage dependent (ingestion of bradyzoites has a shorter prepatent period than ingestion of sporozoites) but is not dose dependent.14 In addition, transmission of T. gondii is most efficient when cats consume tissue cysts (carnivorism) and when intermediate hosts consume oocysts (fecal-oral transmission). Infection of rodents with T. gondii leads to clinical signs of altered behavior, so that the rodent becomes less fearful of cats.15 This may increase the likelihood that the definitive host (cat) will become infected and potentiate the sexual phase of the organism. Whether clinical toxoplasmosis develops is dependent on both host and parasite effects. Some strains of T. gondii may be more pathogenic than others, and some strains may have specific tissue affinities, such as a tendency to cause ocular disease in cats.16,17 If a poor immune response is mounted after primary infection, overwhelming tachyzoite replication that results in tissue necrosis is the major cause of disease.2–4 This mechanism is also likely in cats or dogs with chronic (latent) toxoplasmosis that then become immune suppressed. One example is activation of T. gondii infection in cats or dogs after administration of immunosuppressive drugs such as cyclosporine.18–21 Other immunosuppressive conditions such as FIV infection can also result in activation of toxoplasmosis.22 The mechanisms for chronic clinical toxoplasmosis have not been fully determined.2 T. gondii antigens and antigen-containing immune complexes have been documented in the serum of affected cats. Persistent infection was not associated with chronic kidney failure in cats of one study.23–25 The large majority of cats infected with T. gondii never develop detectable clinical abnormalities. In general, the enteroepithelial cycle in the cat rarely leads to clinical signs. Only 10% to 20% of experimentally inoculated cats develop self-limiting, small bowel diarrhea for 1 to 2 weeks following primary oral inoculation with T. gondii tissue cysts; this is presumed to be from the enteroepithelial replication of the organism. T. gondii enteroepithelial stages were found in intestinal tissues from two cats with inflammatory bowel disease that had positive response to administration of anti-Toxoplasma drugs.26 Eosinophilic fibrosing gastritis was recently described in a T. gondii–infected cat.27 Fatal extraintestinal toxoplasmosis in cats can develop from overwhelming intracellular replication of tachyzoites following primary infection; hepatic, pulmonary, CNS, and pancreatic tissues are commonly involved.2–4,28–30 Kittens infected by the transplacental or transmammary routes develop the most severe signs of extraintestinal toxoplasmosis and generally die of pulmonary or hepatic disease. Common clinical signs in cats with disseminated toxoplasmosis include lethargy, anorexia, and respiratory distress. Disseminated toxoplasmosis has been documented in cats concurrently infected with FeLV, FIV, or feline infectious peritonitis virus, as well as following cyclosporine administration for allergic dermatitis, immune-mediated disorders, or after renal transplantation.18,19,31 Chronic toxoplasmosis occurs in some cats and should be on the differential diagnosis list for cats with uveitis, chorioretinitis (Figure 72-4), cutaneous lesions, fever, muscle hyperesthesia, myocarditis with arrhythmias, weight loss, anorexia, seizures, ataxia, icterus, diarrhea, respiratory distress, or pancreatitis.2–4,32–42 Toxoplasmosis appears to be a common infectious cause of uveitis in cats; anterior uveitis or posterior uveitis can occur, and the manifestations can be either unilateral or bilateral.2,41,42 Kittens infected transplacentally or through the transmammary route commonly develop ocular disease.16 In dogs, respiratory, gastrointestinal, or neuromuscular infection with associated signs of fever, vomiting, diarrhea, respiratory distress, ataxia, seizures, and icterus occurs most commonly with generalized toxoplasmosis.2,43–50 Generalized toxoplasmosis is most common in immunosuppressed dogs, such as those with canine distemper virus infection or those treated with cyclosporine to prevent renal transplant rejection. Neurologic signs depend on the location of the primary lesions and include ataxia, seizures, tremors, cranial nerve deficits, paresis, and paralysis. Dogs with myositis present with weakness, stiff gait, or muscle atrophy. Rapid progression to tetraparesis and paralysis with lower motor neuron dysfunction can occur. One study associated T. gondii antibodies with polyradiculoneuritis in dogs.49 Some dogs with suspected neuromuscular toxoplasmosis probably have neosporosis. Myocardial infection with ventricular arrhythmias occurs in some infected dogs. Retinitis, anterior uveitis, iridocyclitis, nodular conjunctivitis, and optic neuritis occur in some dogs with toxoplasmosis, but they are less common than in cats.50 Cutaneous disease also has been reported, which can manifest as pustules, pruritus, subcutaneous nodules, and/or alopecia.21,51 Diagnosis of toxoplasmosis requires interpretation of specific microbiologic tests (Table 72-1) in light of the presence of consistent clinical abnormalities. No clinical abnormalities are specific for the disease.2–4 TABLE 72-1 Microbiologic Assays for Diagnosis of Toxoplasma gondii Infection ∗Results of serum antibody assays are combined with clinical findings to aid in making a diagnosis of clinical toxoplasmosis. T. gondii should be on the differential diagnosis list for cats or dogs with appropriate clinical findings and nonregenerative anemia, neutrophilic leukocytosis or neutropenia, lymphocytosis or lymphopenia, monocytosis, and/or eosinophilia. Depending on the organ system involved, cats with clinical toxoplasmosis may have increases in serum protein and bilirubin concentrations, as well as increased activities of creatine kinase, ALT, ALP, and lipase.2–4 Findings are similar for dogs; dogs with chronic toxoplasmosis may develop hyperglobulinemia that is generally polyclonal.52 Proteinuria and bilirubinuria have been detected in some dogs or cats with clinical toxoplasmosis. Pulmonary toxoplasmosis most commonly causes diffuse interstitial to alveolar patterns (Figure 72-5); pleural effusion has rarely been documented as well. FIGURE 72-5 Thoracic radiographs of a 7-year-old female spayed domestic shorthair cat with pulmonic toxoplasmosis that developed one month after renal transplantation. There is a diffuse interstitial pattern. The cat subsequently developed respiratory arrest and tachyzoites were seen in fluid that was suctioned from the endotracheal tube used for resuscitation. Necropsy revealed severe, diffuse, necrotizing pneumonia with intralesional tachyzoites. Histiocytic, lymphoplasmacytic, and necrotizing lesions with intralesional tachyzoites were also seen in the renal allograft, ureter, bladder, liver, pancreas, peritoneum, muscularis mucosa of the stomach, and myocardium. Tachyzoites were not seen in the brain, but there was perivascular inflammation that suggested the possibility of infection. Immunohistochemistry confirmed that the tachyzoites were T. gondii. MRI findings in dogs and cats with toxoplasmosis may be unremarkable, or focal or mass lesions may be identified in the brain and/or spinal cord. On MRI, lesions are often isointense on T1-weighted imaging and hyperintense on T2-weighted imaging, and they may enhance peripherally or uniformly with contrast (Figure 72-6).53–55 FIGURE 72-6 T1-weighted postcontrast sagittal magnetic resonance image from an 11-year-old female spayed domestic longhair that was evaluated for a 5-day history of inappetence, ataxia, abnormal vocalization, and circling. The cat was FIV and FeLV negative and had no other immunosuppressive conditions. A contrast-enhancing lesion is present in the right brainstem. CSF analysis revealed a mixed monocytic (primarily lymphocytic) pleocytosis with a total nuclear cell count of 36 cells/µL and a total protein concentration of 78 mg/dL. Meningoencephalitis secondary to T. gondii infection was confirmed at necropsy. Using cytologic examination, T. gondii bradyzoites or tachyzoites can be detected in tissues, effusions, bronchoalveolar lavage fluids, aqueous humor, or CSF (Figure 72-7).32,35,36,38 A definitive antemortem diagnosis of toxoplasmosis can be made if the organism is identified; however, this is uncommon, particularly in association with sublethal disease. In the dog, N. caninum tachyzoites cannot be distinguished from those of T. gondii on cytologic examination, and so further diagnostics are needed to make a definitive diagnosis.2 FIGURE 72-7 A, Tachyzoites of Toxoplasma gondii from the peritoneal cavity of a cat with fulminant toxoplasmosis. Unstained specimen. B, Cluster of tachyzoites in a tracheobronchial lavage specimen from a 12-year-old male neutered cat that developed respiratory distress 3 weeks after renal transplantation. Wright’s stain, 1000× oil magnification. T. gondii oocysts measure 10 × 12 µm. When identified in feces of cats with diarrhea, this suggests infection by T. gondii.2–4,56 However, Besnoitia and Hammondia infections of cats produce morphologically similar oocysts. If oocysts of this size are detected in feces of dogs, N. caninum infection is most likely if clinical disease is present.2 However, T. gondii oocysts can be present if the dog has ingested infected feline feces. PCR assays are available to differentiate T. gondii and N. caninum. Alternately, serum antibody responses can be followed sequentially to attempt to determine the infecting genus. Overall, detection of oocysts in cats has a poor negative predictive value for clinical toxoplasmosis, because most clinical toxoplasmosis results from disseminated infections that occur after oocyst shedding has been completed.
Toxoplasmosis
Etiologic Agent and Epidemiology
Clinical Features
Diagnosis
Laboratory Abnormalities
Serum Chemistry Profile
Urinalysis
Diagnostic Imaging
Advanced Imaging
Microbiologic Testing
Fecal Flotation
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Toxoplasmosis
Only gold members can continue reading. Log In or Register to continue