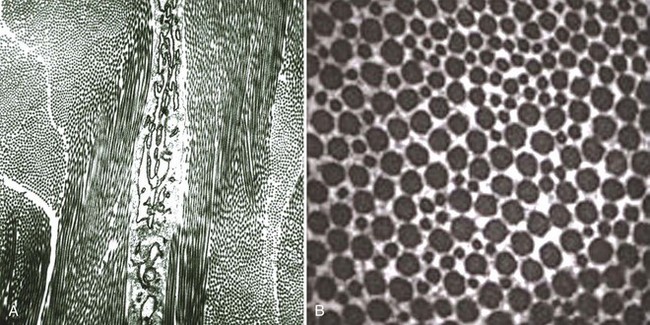
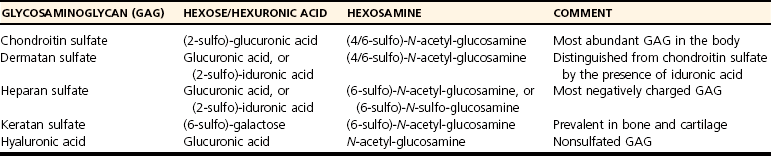
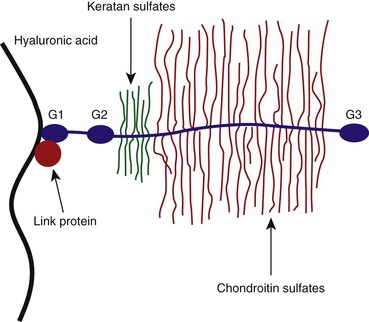
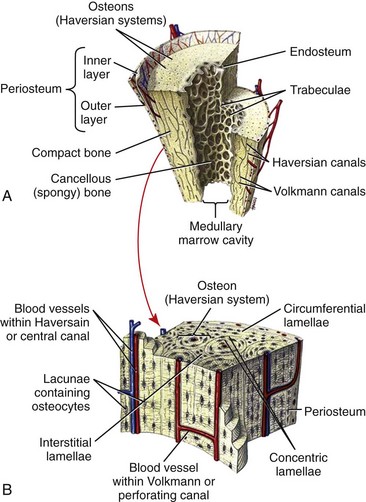
Chapter 40 In vertebrates, the musculoskeletal system is composed of specialized connective tissues that together dictate general body form, and that provide the basic infrastructure of movement and locomotion. In addition, the axial skeleton and rib cage provide protective encasements for the central nervous system and thoracic viscera, respectively. The skeleton is also a repository for mineral and plays an integral role in calcium homeostasis. Postnatal hematopoiesis takes place predominantly within the medullary cavities of long bones, and recent work has disclosed complex and important regulatory interactions between bone-lining cells and elements of the hematopoietic system, indicating that the osseous hematopoietic “niche” is more than simply an anatomic compartment.29,38,61 Skeletal muscle, although not often considered a connective tissue per se, nevertheless accounts for a large proportion of the musculoskeletal system, and plays important roles in amino acid and glucose metabolism and thermoregulation, in addition to its locomotory function. Classically, connective tissues are described as being composed of cells and fibers suspended within an amorphous ground substance. A contemporary view describes them as hypocellular tissues containing tissue-specific mesenchymal cells within a specialized extracellular matrix. Within the musculoskeletal system, connective tissue cells are named in accordance with the tissues they inhabit (e.g., osteoblasts within bone, chondrocytes within cartilage), and these cells have tissue-specific phenotypes and morphologies. Connective tissues may also contain small numbers of migratory cells such as mast cells, macrophages, and other leukocytes. Collagen, the most abundant protein in the body, is a ubiquitous component of connective tissues and was originally identified as discrete fibers with a characteristic banded pattern on microscopic examination (Figure 40-1). The amorphous “ground substance,” originally described as rich in mucopolysaccharides, is a complex and variably hydrated mixture of proteoglycans, other nonfibrillar proteins, and a variety of peptide growth factors, proteolipids, and glycoproteins. Glycoproteins are polypeptides that contain covalently linked carbohydrates. Proteoglycans are one class of glycoproteins in which the carbohydrate conjugates are unbranched polysaccharides containing amino sugars (glycosaminoglycans). The structures of the musculoskeletal system are predominantly of mesodermal origin; the cells of the musculoskeletal system are thus predominantly mesenchymal. A prominent exception is the nucleus pulposus of the intervertebral disc, which is of neuroectodermal (notochordal) origin. In the adult, many musculoskeletal structures contain small but significant populations of progenitor cells (mesenchymal stem cells) that retain a less differentiated and multipotent phenotype. These cells, including the cells of the preosteoblast lineage of bone, or the satellite cells of muscle, play important roles during the healing and regeneration of tissues after injury. Much interest is currently focused on the use of such progenitor cells for tissue engineering, and for the regeneration of tissues such as articular cartilage, which otherwise have limited ability to heal or regenerate after injury.23,80,92 Mechanosensitivity is a common property of connective tissue cells. Mechanotransduction, the process by which cells mount specific and appropriate biologic responses to mechanical stimuli, underlies many aspects of growth and functional adaptation of musculoskeletal tissues.32,62,93 Functional loads on the musculoskeletal system produce distributions of stress (force carried for a given area) and strain (deformation produced in a given direction) within tissue. Adaptation refers to the ability of tissues to actively maintain specific properties such as stiffness and strength that are required for their function and integrity. The response of tissue to stress and strain may be physiologic or pathologic, depending on the prevailing anatomic conditions and the nature of the forces involved. Physiologic response occurs during the formation and remodeling of bone, either during growth or in the context of fracture healing; pathologic adaptation might involve the thickening and fibrosis of a joint capsule in response to chronic joint instability. Under physiologic conditions, mechanical loads may be considered trophic for musculoskeletal tissues, as demonstrated by the atrophy that occurs following tissue disuse or immobilization. Under given conditions of mechanical loading, the cellular response underlies changes in the composition of the extracellular matrix that result in functional adaptations in stiffness, strength, and directional dependence of the tissue. The mechanisms by which mechanical forces activate intracellular signal transduction pathways and drive downstream alterations in gene expression or other cellular behaviors are complex and incompletely understood. Fluid shear along the cell surface can be detected by a variety of cell surface mechanoreceptors, including the primary cilium.12,57,95 Deformation of the plasma membrane may induce conformational changes in transmembrane ion channels or receptors, and may initiate signaling through alteration of transmembrane ion conductance, ligand-independent activation of specific receptors, or shifts in the kinetics of ligand–receptor interactions.76 Direct strain-induced alterations of cytoskeletal protein conformation may also initiate signaling activities by exposing cryptic protein interaction motifs or enzymatic activities.85 Within the musculoskeletal system, forces are usually borne primarily by the extracellular matrix of specific tissues, and cellular responsiveness to deformation of the extracellular matrix is likely to underlie most processes of tissue adaptation.59 Cellular interconnectedness is another general feature of all musculoskeletal tissues, with the exception of cartilage. Immunohistochemical studies of bone, tendon, and ligament have shown that cells are regularly distributed throughout the extracellular matrix and are extensively interconnected through cytoplasmic extensions and intercellular gap junctions.16,17,88 This system of intercellular connections presumably forms the basis for intercellular communication, and although the significance of this aspect of tissue organization is not completely understood, this phenomenon is thought to underlie the ability of cells to orchestrate regional adaptive tissue responses to mechanical or biologic stimuli.42 These intercellular connections are reestablished during the regeneration of tissues such as bone. In contrast, in tissues such as tendons and ligaments in which healing occurs through fibrosis, regional connectivity among scar fibroblasts is not effectively reestablished.44 This is likely to be related to the inferior mechanical properties and relative lack of adaptability of the scar, as compared with the native tendon or ligament. All collagens share a common tertiary structure, consisting of a triple helix made up of three separate polypeptide molecules called alpha chains. Alpha chains are encoded by unique genes, of which at least 30 are present in mammals. Homotypic collagens, such as types II and III, are composed of three identical alpha chains, and heterotypic collagens, such as type V, may be composed of up to three different alpha chains. Additional forms of collagen arise from the use of alternative transcription start sites or alternative splicing of primary alpha chain gene transcripts, leading to a large repertoire of individual collagen molecules with diverse structural, mechanical, and functional properties.54 Alpha chain gene products are variable-length polypeptides with a major central region consisting of (Gly-X-Y)n repeats, flanked by shorter globular N-terminal and C-terminal telopeptide domains. Collagen biosynthesis commences with transcription of the alpha chain gene and translation of the alpha chain mRNA, leading to the formation of pre-pro-alpha chains, or pre-pro-collagen. The activity of prolyl- and lysyl-hydroxylases leads to hydroxylation of specific lysine and proline residues within the polypeptide; these reactions occur concurrently with translation of the mRNA and require ascorbic acid, oxygen, and iron as co-factors. Proline hydroxylation is an essential and primary rate-limiting step in collagen biosynthesis, as demonstrated in animals with loss of function mutations in elements of the prolyl-3-hydroxylase enzyme complex that develop a severe form of osteogenesis imperfecta.48 Other posttranslational modifications of pre-pro-alpha chains include O-linked glycosylation reactions. Assembly of the triple helix occurs intracellularly and is regulated by interactions between C-terminal telopeptides of pre-pro-alpha chains. Triple helices are stabilized by disulfide bonds, and folding is facilitated by numerous molecular chaperones, as well as by cis-trans-isomerization of peptide bonds, a reaction catalyzed by prolyl-peptidyl isomerase. These reactions result in the formation of triple helical procollagen. Finally, the globular telopeptide domains are cleaved by a wide variety of metalloproteinases shortly before or after secretion, resulting in the formation of tropocollagen.34 The assembly of tropocollagen into macromolecular fibrillar complexes within the extracellular matrix is referred to as fibrillogenesis, and occurs as tropocollagen molecules align in a staggered pattern and are covalently cross-linked into mature fibrils by the activity of copper-dependent lysyl-oxidases. Fibrils vary in diameter from 50 to several hundred nM in diameter. Self-assembly of tropocollagen is thought to be largely entropic, reflecting the tendency of hydrophobic residues of tropocollagen to escape the aqueous environment by associating with the interior of a developing fibril.39 Nascent fibrils interact with a wide variety of proteoglycans, particularly the small leucine-rich proteoglycans (see later), and several of these proteoglycans appear to play key roles in the regulation of fibril diameter and interfibrillar interactions.5 Fibrils may be further assembled into fascicles. In some tissues, such as tendon and ligament, fascicles are further bundled into macroscopic fibers, with diameters up to several hundred µM (Figure 40-2). Proteoglycans are a large and structurally diverse group of glycosylated proteins that are ubiquitous and important components of the extracellular matrix of all connective tissues. The basic structure of a proteoglycan consists of a core protein with one or more covalently attached glycosaminoglycan side-chains (Figure 40-3). The core proteins are encoded by unique genes and represent a wide variety of protein forms and sizes. The glycosaminoglycans are variable-length polymers of repeating disaccharide units; individual glycosaminoglycans are defined by the specific disaccharide unit of which they are composed (Table 40-1). Glycosaminoglycans may be divided into two major classes: glucosaminoglycans (heparan sulfate and keratan sulfate) contain D-glucosamine, and galactosaminoglycans (chondroitin sulfate and dermatan sulfate) contain D-galactosamine. The hexosamine units within glycosaminoglycans may be extensively sulfated, which renders them highly anionic and hygroscopic. Hyaluronic acid (hyaluronan) is a nonpeptide, conjugated, nonsulfated glycosaminoglycan consisting of repeating units of D-glucuronic acid and D-glucosamine. Proteoglycans may be divided into two major families. The large aggregating proteoglycans include aggrecan and versican and consist of a backbone of hyaluronic acid with multiple noncovalently linked proteoglycans; the attachment of individual proteoglycans to the hyaluronic acid molecule is mediated by a link protein that binds simultaneously to the hyaluronic acid backbone and to the globular N-terminal region (G1 domain) of the core protein of proteoglycan (see Figure 40-3). Aggregating proteoglycans are massive and highly anionic branched macromolecular assemblies that have a high affinity for water. In many types of extracellular matrix, this affinity yields a high level of hydration and turgidity, which is critical for the compressive properties of the tissue. In particular, articular cartilage relies on the incompressible nature of water to provide energy absorption and resistance to large joint loads. The small leucine-rich proteoglycan family contains 17 members. The major small leucine-rich proteoglycans include biglycan, decorin, fibromodulin, and lumican. The small leucine-rich proteoglycans have multidomain core protein structures with conserved N-terminal cysteine-rich regions, characteristic leucine-rich repeats, and variable patterns of glycosaminoglycan conjugation. Patterns of small leucine-rich proteoglycan expression are tissue specific and play diverse roles in the regulation of collagen fibrillogenesis and elastogenesis, as well as in the modulation of growth factor signaling to connective tissue cells.5,72 Elastic fibers are common components of extracellular matrix that provide flexibility, extensibility, and resilience to tissues. Elastic fibers are present in many musculoskeletal tissues in variable quantities, depending on tissue requirements for elasticity. Thus, elastic fibers make up the majority of the nuchal ligament and account for less than 5% of the dry weight of tendon.86 The formation of elastic fibers (elastogenesis) is an important and active process during development and growth. However, once formed, elastic fibers are extremely stable, and in the adult, turnover of elastic fibers is negligible.58 Elastogenesis is reactivated during the reparative phase of tissue injury and can be induced in a variety of tissues by sustained tensile mechanical stress such as occurs during distraction osteogenesis.36 Elastic fibers are composed mainly of elastin, an insoluble and extensible protein biopolymer. The monomeric component of elastin is the protein tropoelastin, a highly conserved peptide with a molecular weight of 65 to 70 kDa. Vertebrates possess a single tropoelastin gene; however, the primary gene transcript undergoes extensive alternative splicing, resulting in the production of a variety of forms of tropoelastin.37 Following synthesis, tropoelastin is secreted and delivered to developing elastic fibers as a complex with a specific elastin binding protein. Incorporation of tropoelastin into a nascent elastic fiber involves the processes of coacervation and cross-linking.55 Coacervation refers to the temperature-dependent alignment and macromolecular assembly of tropoelastin monomers. Self-assembly of tropoelastin is inhibited at low temperatures; however, at physiologic temperatures, conformational changes are induced that expose hydrophobic domains within the tropoelastin monomers and that facilitate stable association between monomers based on hydrophobic interactions. Following self-assembly, elastin polymers are covalently cross-linked and stabilized by members of the copper-dependent family of lysyl oxidases. The formation of elastin requires the presence of both fibrillin microfibrils and fibronectin within the extracellular matrix; these form a scaffold for the deposition of tropoelastin.20 Mature elastic fibers consist of a core region of cross-linked elastin polymer surrounded by an outer mantle of fibrillin-rich microfibrils. The requirement of fibrillin for elastogenesis is demonstrated in human patients with Marfan syndrome, in which loss of function mutations in the gene for fibrillin-1 lead to defective elastogenesis, resulting in arterial wall defects and pathologic laxity of multiple joints.75 Elastic fibers can undergo remarkable elastic deformation or strain of approximately 70% of their resting length, with a maximum extension of 220% before loss of strength. Elastin has a relatively low modulus of elasticity (300 to 600 kPa) compared with that of type I collagen (≈106 kPa). Yet, below maximum extension, elastic fibers are capable of undergoing billions of cycles of extension and relaxation without loss of integrity.40 The molecular mechanisms underlying the elasticity of elastin are thought to be predominantly entropic. In the relaxed state, the organization of tropoelastin is highly disordered. Fiber elongation that occurs with tensile stress causes the tropoelastin molecules to become aligned and more highly ordered; elastic recoil thus represents the inherent tendency of the macromolecular structure to return to a state of maximum entropy.55 In addition to their structural role within the extracellular matrix, elastic fibers are capable of modulating the behavior of connective tissue cells through binding and activation of specific cell surface integrins and elastin receptors. Elastin-mediated signaling can modulate diverse cellular processes such as survival and proliferation, migration, and chemotaxis.66 Despite its inherent stability, elastin is susceptible to proteolytic cleavage by a diverse group of elastases. Neutrophils are a common source of elastase activity, and elastic fiber degradation occurs commonly in the setting of acute inflammation. As with many components of the extracellular matrix, proteolysis of elastic fibers can produce small bioactive peptide fragments of elastin (elastokines) that can exert local or distant chemotactic, proliferative, or proinflammatory effects.21 The extracellular matrix of connective tissue contains numerous other proteins, proteolipids, and glycoproteins. Despite their low abundance, these elements play important roles. The fibrillin family consists of fibrillins-1, -2, and -3, along with a related group of proteins called the latent transforming growth factor (TGF)-β–binding proteins (LTBPs), of which there are four. The fibrillins are large calcium binding proteins that adopt elongated, rod-like secondary structures and form fibrillar macropolymers stabilized by transglutaminase-mediated cross-links. They serve important structural roles in connective tissue through their interaction with proteoglycans, collagen, and, particularly, elastin.18,63 Also, in conjunction with the LTBPs and several of the small leucine-rich proteoglycans (SLRPs), the fibrillins bind a number of extracellular matrix-associated growth factors such as TGF-β and modulate the bioactivity of these factors by regulating their interaction with their cognate receptors on cells.35 Fibronectin is a large multidomain glycoprotein that is a ubiquitous component of extracellular matrix and that functions as an important substrate for cell adhesion. Fibronectin functions as an initial scaffold for the deposition of other extracellular matrix structures such as fibrillin microfibrils, elastin, and collagens. Other extracellular matrix components include growth factors, several bone-specific proteins (osteonectin, osteocalcin, bone sialoprotein), and a wide variety of glycoproteins such as the microfibril-associated glycoproteins (MAGPs), which regulate the assembly of fibrillar elements of the extracellular matrix. The skeleton forms the basic frame that supports the locomotory apparatus and from a mechanical point of view consists largely of a series of lever arms designed to counteract the force of gravity and to constrain and direct the forces of muscular contraction. Bones vary greatly in shape among species, but their molecular architecture is highly conserved. In adults, bone occurs predominantly in two forms. Cortical, compact, or osteonal bone is composed of longitudinally oriented, overlapping, cylindrical Haversian units that are made up of a central Haversian canal surrounded by concentrically arranged bone lamellae (Figure 40-4). Trabecular, spongy, or cancellous bone is located primarily in the metaphyseal regions or is projected into the medullary canal; it consists of thin and interconnected rods, plates, and struts called trabeculae (Figure 40-5). The inner and outer surfaces of a bone are covered with a thin endosteal or periosteal membrane containing bone-lining cells, osteoprogenitors, and osteoblasts. The periosteal extracellular matrix is rich in type I collagen, along with a variety of proteoglycans and appreciable quantities of elastin.
Tissues of the Musculoskeletal System
General Organizational Features of Connective Tissues
General Features of Cells of the Musculoskeletal System
Components of the Extracellular Matrix
Proteoglycans
Elastin and Elastic Fibers
Other Components of the Extracellular Matrix
Composition and Properties of Specific Connective Tissues
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Tissues of the Musculoskeletal System
Only gold members can continue reading. Log In or Register to continue