Chapter 40 Tissue Cyst-Forming Coccidia of Marine Mammals
Parasite prevalence
Toxoplasma gondii
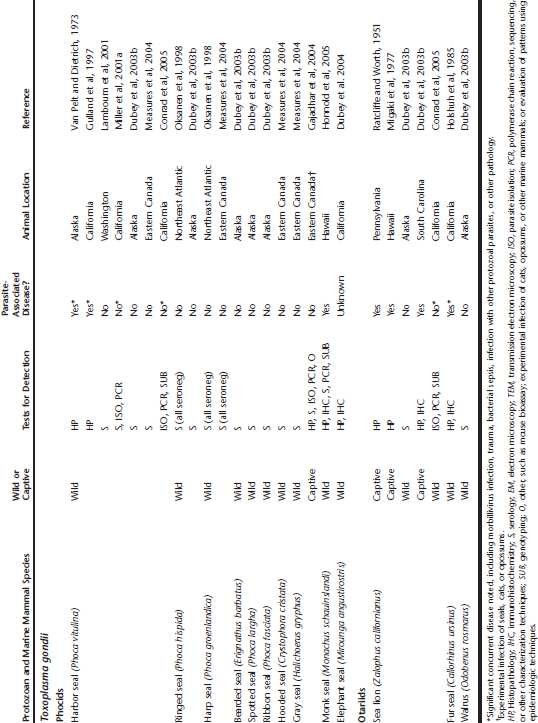
The main tissue cyst-forming coccidia reported from marine mammals are Toxoplasma gondii, Sarcocystis neurona, and unidentified Sarcocystis spp. parasites. The earliest report of T. gondii infection in marine mammals dates back 55 years to a captive sea lion residing in Pennsylvania.72 Since that time, T. gondii has been reported from wild and captive marine mammals from North America, Hawaii, South America, Europe, Asia, and Australia Table 40-1. Based on the global distribution of T. gondii in cats, it is reasonable to suspect that marine mammals in other areas are also exposed.
Table 40-1 Toxoplasma gondii, Sarcocystis neurona, Sarcocystis spp., and Unidentified Protozoan Parasite Exposure in Marine Mammals
Even arctic- and subarctic-dwelling seals and walruses (Odobenus rosmarus) with low exposure to cats have serologic evidence of T. gondii infection, prompting concerns regarding consumption of uncooked marine mammal tissue by indigenous human populations.30,32,50 Other subarctic species, such as Alaskan sea otters (Enhydra lutris kenyoni), have a lower prevalence of infection by T. gondii than their southern counterparts (Enhydra lutris nereis).36,61
A wide range of marine mammals has been shown to be infected, including otariids, phocids, cetaceans, mustelids, and sirenians (see Table 40-1). However, the significance of the tissue cyst-forming protozoa to some marine mammal populations was not fully appreciated until the past decade. Based on current diagnostic testing, at least 36% of southern sea otters presenting for necropsy are infected with T. gondii, and 60% or more are seropositive.14,30,61,62 Protozoal infections caused by T. gondii and S. neurona are also a major cause of otter death, responsible for 8.5% to 23% of southern sea otter mortality.42,78
Reports of T. gondii infection in marine mammals range from incidental to severe and fatal, and striking differences are noted when the various studies are organized by marine mammal taxa. Clinically significant T. gondii infections are reported most frequently from southern sea otters and odontocete cetaceans. In contrast, T. gondii–associated disease is uncommon in otariids and phocids and is often associated with concurrent disease or immunosuppression.34,37,81 This low prevalence of T. gondii–related pathology is unlikely to result from low surveillance because rehabilitation and necropsy programs for otariids and phocids have existed worldwide for many years. However, mild or asymptomatic infections are easily missed.
Sarcocystis neurona
Sarcocystis neurona, the major causative agent of equine protozoal myeloencephalitis (EPM), was first reported as a pathogen of marine mammals over the past decade.* In contrast to T. gondii, reports of confirmed S. neurona–associated marine mammal mortality have thus far been limited to the Pacific Coast of North America (see Table 40-1), in areas where Virginia opossums (Didelphis virginiana) have been introduced. The reasons for this localized occurrence of infection are unclear because opossums capable of shedding S. neurona sporocysts are present throughout much of North and South America. Marine mammals with concurrent T. gondii and S. neurona infections are often reported from the Pacific Coast of North America, suggesting high levels of environmental exposure to these potential pathogens.14,48,59
Fatal infections by S. neurona are common in Pacific harbor seals (Phoca vitulina richardsi) and southern and northern sea otters from California, Washington, and Oregon.* This parasite is one of the more common causes of harbor seal mortality at the largest marine mammal rehabilitation facility in California, affecting primarily adult and subadult seals.12,13,45,59 In California, S. neurona is a major cause of wild sea otter mortality, causing severe meningoencephalomyelitis, lymphadenopathy, pneumonia, musculoskeletal and cardiovascular disease; this parasite appears to be increasing as a cause of sea otter death.42,43,48,56,60 A single wild cetacean from coastal California with possible S. neurona infection has also been recognized (see Table 40-1).
In contrast, T. gondii– or S. neurona–related mortality has not been reported from Alaskan or Russian (Enhydra lutris lutris) sea otters, although seropositive Alaskan otters have been detected.30 Similarly, no sirenians or otariids have been reported with S. neurona infection, although they inhabit coastal areas where opossums reside in North and South America. Reports of asymptomatic S. neurona infections are absent from the marine mammal literature. This is undoubtedly a result of (1) underreporting of incidental cases and (2) difficulty distinguishing S. neurona tissue cysts from related, presumably less pathogenic, Sarcocystis species on histopathology.
Other Sarcocystis spp. Infecting Marine Mammals
Reports of Sarcocystis spp. infection in marine mammals span over 130 years and encompass more than 14 species, including otariids, phocids, cetaceans, mustelids, ursids, and sirenians (see Table 40-1). The first report of any tissue cyst-forming coccidial infection in a marine mammal was described by Huet39 in 1882 from skeletal muscle of a sea lion that died in captivity in France (see Table 40-1). Huet attributed the death of this animal to lungworm infection, although intramuscular tissue cysts were grossly visible at necropsy. The only reported infection of a baleen whale by tissue cyst-forming coccidia is Sarcocystis spp. tissue cysts noted incidentally in skeletal muscle of a sei whale (Balaenoptera borealis) harvested for human consumption.1
Little is known about the identity, host range, or definitive hosts for these parasites. However, some have been distinguished from S. neurona based on the disease presentation, parasite ultrastructure, immunohistochemistry, molecular characterization, and parasite distribution in host tissues.26,51,74,83 Reports of Sarcocystis spp. parasitism excluding S. neurona tend to fall into one of two major groups: (1) animals with tissue cysts (sarcocysts) noted incidentally in skeletal or cardiac muscle at necropsy, accompanied by variable, often minimal chronic infection, and (2) animals with severe necrotizing hepatitis associated with proliferation of intermediate parasite stages.
Muscle sarcocysts have been reported incidentally from sea otters, river otters, phocids, otariids, mysticetes, and odontocetes (see Table 40-1). Definitive speciation is not possible by conventional histopathology; with transmission electron microscopy (TEM); however, marked differences are often apparent, particularly in the structure of the cyst wall.26 Given the large range of Sarcocystis spp. reported in terrestrial animals, it is likely that numerous species infect marine mammals, possibly including marine-adapted species. Limited molecular and ultrastructural characterization has suggested that these Sarcocystis spp. sarcocysts are distinct from S. neurona, but may be present concurrently with S. neurona in some animals, making accurate confirmation of infection at the light microscopic level a challenge.26,48
Sarcocystis spp. parasites associated with fatal necrotizing hepatitis are reported from captive California sea lions (Zalophus californianus), a monk seal (Monachus schauinslandi), two polar bears (Ursus maritimus), and a wild striped dolphin (Stenella coeruleoalba) (see Table 40-1). In these animals, protozoal merozoites proliferate in the liver, causing severe hepatic damage. Some reports tentatively identify these parasites as Sarcocystis canis, although further characterization is needed.30,74,83
Other Tissue Cyst-Forming Protozoa Infecting Marine Mammals
An unidentified protozoan was reported in 1994 from a Pacific harbor seal from Northern California.46 Histopathology revealed necrotizing meningoencephalitis with intralesional protozoal tachyzoites. This parasite is antigenically distinct from T. gondii, S. neurona, and Neospora caninum and remains unidentified.
High seroprevalence of some marine mammals, especially cetaceans, for N. caninum has been reported,30 but no confirmed N. caninum infections have been reported in marine mammals. More than 500 necropsied California sea otters have been screened since 1998 with histopathology, immunohistochemistry (IHC), parasite isolation, and polymerase chain reaction (PCR); all have proved negative thus far for N. caninum,57 although neosporosis is a significant problem in California dairy cattle. Canids are the definitive hosts for N. caninum. Rarely, southern sea otters with high antibody titers to T. gondii exhibit low, presumably cross-reactive titers to N. caninum, but they are negative for N. caninum on histopathology, IHC, and PCR. However, serologic cross-reactivity with T. gondii does not appear to be a factor in some cases. In one recent study, titers to N. caninum were detected in marine species that occurred in the absence of a positive titer for T. gondii.30 This finding is intriguing and could be substantiated with confirmation of N. caninum infection in marine mammals in the future. Significant potential also exists for serologic cross-reactivity with other, as-yet unrecognized protozoa. Caution is advised when evaluating serologic tests in marine mammals; efforts to authenticate the results using more specific tests are warranted.
INDIRECT EFFECTS OF PROTOZOAL INFECTION AND DISEASE
In addition to death from meningoencephalitis and other systemic disease, potential indirect causes of mortality exist for T. gondii– and S. neurona–infected marine mammals, including increased susceptibility to trauma.6,42 Negative impacts of T. gondii brain infection on mentation and behavior are also reported in humans, laboratory animals, and experimentally infected gray seals.14,32 Reproductive impacts, such as fetal death or abortion from transplacental T. gondii infection, may prove to be important for some populations of marine mammals, particularly cetaceans.41,73 In addition, cardiovascular, pulmonary, lymphoreticular, and musculoskeletal pathology caused by T. gondii and S. neurona infection is reported from many species of marine and terrestrial animals. Protozoal-associated cardiac pathology may be challenging to distinguish from that caused by other agents because (1) affected animals often strand late in the course of the disease, when cardiac damage is extensive, and (2) more than one agent may be associated with cardiac pathology; for example, protozoal infection and domoic acid intoxication may affect animals concurrently.43,56
ETIOLOGIC AGENT AND TRANSMISSION
Toxoplasma gondii
The genus Toxoplasma consists of a single species, T. gondii, with several distinct strains, or genotypes.14,63 Toxoplasma gondii is a single-celled protozoan parasite with a complex life cycle involving both definitive (oocyst-shedding) hosts and intermediate hosts that support the tissue cyst stage of the parasite.77 Virtually any warm-blooded vertebrate, including humans, may serve as intermediate hosts. Thus far, only domestic and wild felids (cats) are known to serve as definitive hosts and support the sexual phase of the parasite’s life cycle, producing millions of infective oocysts that are shed in the cat’s feces (Figure 40-1). New hosts are infected through consumption of tissue cysts in muscle, brain, or other organs of intermediate hosts; by consumption of oocyst-contaminated paratenic hosts; or by accidental consumption of sporulated oocysts derived from the feces of infected cats in soil, in water, or on vegetation, or transplacentally.77 Transplacental transmission is also well documented.77 The interval between parasite ingestion by cats and oocyst shedding may be as short as 2 to 3 days when cats ingest T. gondii tissue cysts in the flesh of intermediate hosts.
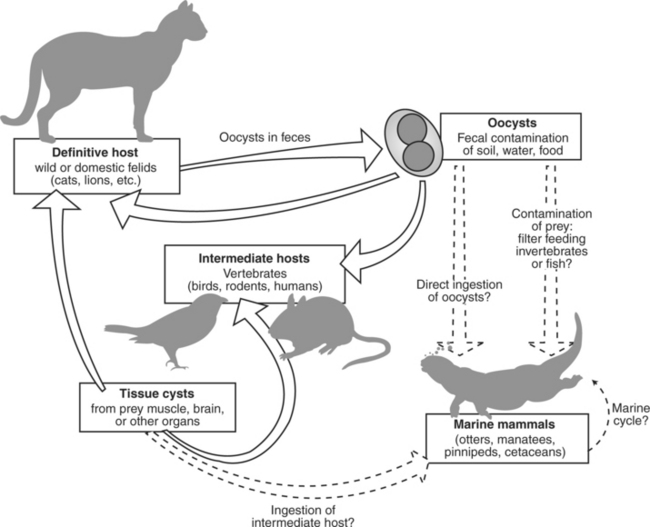
Fig 40-1 Life cycle of Toxoplasma gondii showing potential sources of marine mammal exposure (hatched lines).
Infected cats that are actively shedding oocysts typically exhibit no clinical abnormalities and may pass 100 million infective oocysts in their feces over a 10- to 14-day period.77 Once present in the environment, oocysts become infective within 1 to 5 days and may remain viable for months to years under optimal conditions. Protozoal oocysts (T. gondii and N. caninum) and sporocysts (S. neurona) are resistant to most disinfectants but are susceptible to boiling water, formalin, and iodine. The infective dose may be as low as a single sporulated oocyst.
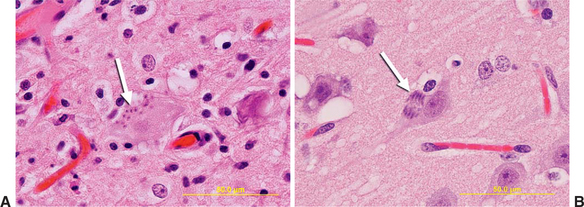
Intermediate hosts for T. gondii include all warm-blooded animals.77 These hosts are infected through consumption of other infected intermediate hosts or oocysts. Once ingested by intermediate hosts, these parasites leave the intestinal tract and spread systemically as invasive, rapidly multiplying, intracytoplasmic tachyzoites. Occasionally these infections are fatal, especially for fetuses, immunosuppressed individuals, or highly susceptible animals, including Australian marsupials and New World monkeys. As specific host immunity is generated, conversion from rapidly multiplying tachyzoites to slower, less active bradyzoites occurs, and bradyzoite-filled tissue cysts develop, primarily in the brain, spinal cord, skeletal muscle, and myocardium (Figure 40-2). Once present, tissue cysts may persist for the life of the host, and infection becomes chronic, perhaps lifelong. Recrudescence of active infection with development of rapidly invasive tachyzoites may occur in chronically infected humans and animals when host immunity wanes.77 For marine mammals, parasite recrudescence with concurrent disease may also be important, especially for T. gondii–infected animals; numerous reports exist in the literature of animals with distemper, sepsis, or other significant disease in addition to toxoplasmosis* (see Table 40-1).
Sarcocystis spp. and S. neurona
The genus Sarcocystis is large and diverse with many uncharacterized species. For well-described species, the life cycles alternate between carnivores or omnivores, and herbivores (e.g., coyote-deer), with the carnivore/omnivore as definitive host and herbivores as intermediate hosts. In contrast to Toxoplasma, the genus Sarcocystis consists of numerous species, each utilizing a different range of definitive and intermediate hosts to complete their life cycle.27 Little is known about the life cycles or host dynamics of Sarcocystis spp. infecting marine mammals, other than a putative link to opossums for S. neurona.30
As noted earlier, S. neurona first gained wide recognition as the primary cause of EPM. The life cycle for this parasite is similar to T. gondii, but with some important differences. New World opossums (Didelphis virginiana and D. albiventris) are the only confirmed definitive hosts for S. neurona. As with cats, opossums appear to be asymptomatic hosts for S. neurona and may shed large numbers of sporocysts without clinical signs. However, unlike T. gondii, prolonged or repeated fecal shedding of Sarcocystis spp. by opossums has been demonstrated. The infective stage for S. neurona is a sporocyst that is immediately infective when defecated.24,30
The intermediate host range for S. neurona includes a wide variety of birds and mammals, excluding humans.24,30 Invasive parasite stages resulting from asexual division of S. neurona are called merozoites, which penetrate host cells and proliferate to form schizonts and later tissue cysts. Sea otters and harbor seals are capable of serving as intermediate hosts, with tissue cyst formation in heart, skeletal muscle, and other tissues and proliferation of merozoites in the brain, lung, and other locations.* Unlike T. gondii, tissue cyst formation has not been reported in the central nervous system for S. neurona–infected animals.
Sarcocystis neurona also differs from T. gondii regarding the dominant pattern of asexual division occurring within host cells, a feature that may be used to distinguish these parasites microscopically and ultrastructurally (see Figure 40-2). Division of T. gondii occurs by endodyogeny, in which two new parasites are formed by lateral division within the cytoplasm of a “mother cell.”24 Schizogony of S. neurona occurs by endopolygeny, in which “daughter” parasites (merozoites) bud off the surface of a mother cell, forming a distinctive, flowerlike radial arrangement called a rosette-form schizont (Figure 40-3).
Neospora caninum
Neospora caninum is closely related to T. gondii and shares some common features in its life cycle, including significant potential for vertical transmission.24 The definitive hosts for N. caninum are canids, and numerous intermediate hosts have been identified, most notably dogs and cattle.24 Neosporosis is a leading cause of abortion in dairy cattle worldwide.24 Vertical transmission is a major route of parasite propagation, and infected cows and dogs may transmit the parasite to their offspring through successive pregnancies.
CLINICAL SIGNS
For many stranded marine mammals with protozoal disease, clinical data are not available because the animals were found dead or died soon after stranding. Incidental or mild T. gondii infections have been identified in phocids, otariids, mustelids, and cetaceans (see Table 40-1). Along the Pacific Coast of North America, reports of live-stranded marine mammals with severe T. gondii– or S. neurona–associated disease are common. Depending on the parasite involved, host age and immunocompetence, concurrent disease, and other factors, clinical signs may be absent or may consist of anorexia, depression, fever, central nervous system (CNS) disease, lymphadenopathy, icterus, abortion, stillbirth, and neonatal mortality.* Neurologic deficits are often the most prominent clinical abnormality and may include blindness, pupillary mydriasis, decreased mentation, sudden and episodic cessation of activity (e.g., eating, grooming), lack of aggression or increased aggression, unusual or repetitive stereotypic behavior, ambulatory and proprioceptive deficits, ataxia, paresis, paralysis, loss of bladder tone, obtundation, tremors, seizures, and coma.45,48,53,66,75 Tremors may worsen with stress, continued activity, or stimulation; may spread from one area (e.g., head, limb) to others, becoming generalized over time; and may be symmetric or asymmetric.
Also common at necropsy of T. gondii– and S. neurona–infected marine mammals is the observation of diffuse, severe lymphadenopathy, sometimes with concurrent splenomegaly and nodular hyperplasia of splenic white pulp.30,42,56 A captive sea lion that died from toxoplasmosis exhibited severe lymphadenopathy, dysphagia, depression, and anorexia before death.30 Reported musculoskeletal deficits include muscle tremors, myalgia, deficits in ambulation or proprioception, paresthesia, and paresis. Possible cardiac deficits include arrhythmias, tachycardia, pulse deficits, and clinical evidence of heart failure (e.g., ascites, generalized congestion, pulmonary edema, pleural effusion).43,58 Some animals exhibit severe T. gondii– or S. neurona–related interstitial pneumonia at necropsy, suggesting that protozoal infection should be considered for animals with diffuse respiratory disease.56 Little clinical information is available for cetaceans or sirenians with protozoal disease because most are recovered dead or die soon after stranding. However, fetal and neonatal death caused by T. gondii appears prominent in cetaceans.41,73
Differentiating between protozoal infections and other systemic disease, or between T. gondii and S. neurona infection is not feasible based purely on clinical signs; both parasites may cause severe disease and may be present concurrently in sick animals.14,49,59 Also, confirmation of infection is not equivalent to confirmation of disease; numerous reports exist of marine mammals with incidental T. gondii and Sarcocystis spp. infections (see Table 40-1).10,56 Careful screening for concurrent disease is also indicated.
Severe, necrotizing hepatitis caused by Sarcocystis parasites that appear distinct from S. neurona are described from polar bears, sea lions, a monk seal, and a striped dolphin, as discussed previously (see Table 40-1). Reported clinical signs included acute anorexia, lethargy, gastrointestinal (GI) illness, and icterus, often with a short course and fatal outcome.33,83
In summary, protozoal infection should be considered for any marine mammal that strands alive with CNS, lymphoreticular (e.g., lymph nodes, thymus, spleen), musculoskeletal, cardiovascular, respiratory, hepatic, or reproductive abnormalities. In the living animal, confirmation of infection is based on demonstration of elevated or rising antibody titers or clinical response to long-term antiprotozoal therapy (see Clinical Therapy). Additional diagnostic procedures, such as muscle biopsy, electrocardiography (ECG), brain magnetic resonance imaging (MRI) or computed tomography (CT), and PCR from serum, muscle, or cerebrospinal fluid (CSF), may be attempted for valuable or rare individuals. Important differential diagnoses for neurologic disease include hypoglycemia, hypothermia, hyperthermia, hyponatremia, hypernatremia, trauma, aberrant parasite migration (larva migrans), other infectious disease (e.g., viral or fungal infection), and exposure to natural or anthropogenic toxins such as domoic acid.80 These agents cause illness that could be confused with systemic protozoal infection or may interact synergistically to produce clinical disease.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree