Fig. 1.
Distribution of degenerating terminals in the neocortex of macaca mulatta following hemispherectomy (from Myers (45)).
2.3.2 Type of Callosal Connections
The corpus callosum fibre organisation is heterogeneous, containing fibres that project to either homotopic or heterotopic sites in the opposite hemisphere. All cortical areas that have contralateral connections send some fibres to homotopic areas. Projections to heterotopic sites such as the primary somatic sensory cortex project not only to the contralateral S1, but also to the neighbouring S2, as well as to the supplementary (SSA) areas (52). Further, the inferior temporal lobule projects not only to the contralateral inferior temporal lobule, but also to the opposite superior temporal sulcus, cingulate gyrus, and parahippocampal area (53). Since these areas also receive ipsilateral projections from the inferior parietal lobule (46, 54–57), it is evident that the contralateral projections parallel ipsilateral cortical projections (53). The contralateral connections are less dense than the ipsilateral ones, where as a rule termination zones in the opposite hemisphere are more restricted than in the ipsilateral hemisphere.
2.3.3 Topological Organisation of the Corpus Callosum
Clinical and experimental evidence indicates that discrete lesions of the forebrain commissures differentially affect cortical functions. These observations are in keeping with the topological organisation of the commissures. For example, in humans tumours of the anterior part of corpus callosum result in a behavioural syndrome similar to schizophrenia, while invading other parts of the callosum does not (58). Similarly, anterior lesions result in certain forms of apraxia, while posterior lesions can result in deficits of reading and writing (59). Further selective lesions of posterior parts of the corpus callosum result in impairments of auditory function in humans (60). In the primate, lesions of both the splenium of the corpus callosum and the anterior commissure selectively disrupt visual functions. However, the visual deficits of the two lesions are qualitatively different (61).
3 Methods of Transection of the Commissures and Optic Chiasma in Split Brain Animals
Techniques of callosal section differ profoundly across different species due to major differences in anatomy and physiology between simple and complex species. In particular, there are profound differences between rodent vision and other mammals. Its nocturnal specialisation obviously make the rat unsuitable for split-brain brain research, involving lateralisation of visual information (see Table 1).
Table 1
Interspecies differences in visual function and anatomical structure
Monkey | Cat | Rabbit | Rat | |
|---|---|---|---|---|
Vision | Diurnal | Crepuscular | Crepuscular | Nocturnal |
Retina | Foveate | Area centralis | Area centralis | Concentric |
Lens | Biconvex | Biconvex | Biconvex | Spherical |
Binocular field | 130° | 100° | 22° | 50° |
Convergence | 35° | 20° | 12° | Not known |
Pursuit movements | Present | Present | Present | None |
Binocular vision | Present | Present | Present | Not known |
Optic nerve fibres | 1,210,000 | 154,000 | 265,000 | 75,000 |
Ipsilateral fibres | 50% | 40% | 10% | 10% |
Acuity (cycles/°) | 64 Hz | 6 Hz | 4 Hz | 0.3 Hz |
Extrastriate areas | 28 | 10 | 6 | None |
3.1 General Considerations
3.1.1 The Newton–von Gudden Principle
The first important anatomical variable concerns the Newton–von Gudden principle. This describes the effect of phylogenic changes in the positioning of the eyes in the head on binocularity, and the proportion of contralateral versus ipsilateral fibres in the optic chiasma (see Table 1). Animals with laterally sited eyes have a reduced binocular field, as well as a corresponding increase in the number of crossed fibre projections in the optic chiasma. This is seen very clearly in the rabbit (which has maximal laterally sited eyes in the mammalian order) and has only 22° of binocularity combined with 90% crossed fibres in the optic chiasma. This contrasts with the monkey’s frontally sited eyes, and 130° of binocularity accompanied by 50% of crossing of fibres in the optic chiasma. This means that transection of the optic chiasma in different split-brain species will have dramatically different effects on the size of the total visual field. In the split-brain monkey, the monocular visual field will be only 50% following section of both the chiasma and callosum as compared with the split-brain rabbit (including chiasma section) with a monocular visual field of 5–6%. The split-brain monkey will have little or no visual learning impairment, following visual field reduction; whereas the rabbit will be forced to acquire and adopt entirely novel visual scanning strategy, without which visual learning is impossible (62).
3.1.2 Presence of a Falx
The presence of a falx in the supracallosal space between the cerebral hemispheres is a critical factor which facilitates callosal section. This is an extension of the dura mater which extends into the space above the corpus callosum between the cerebral hemispheres in certain mammals. It is present in primates, dogs, and cats. It is not present in rabbits, rats or mice. The falx facilitates retraction of the hemisphere without any direct contact with the pia mater and other vasculature of the medial neocortex or cingular cortex. Accordingly in primates, cats, and dogs it is relatively safe to avoid such midline damage with section of the corpus callosum. In animals lacking a falx, such damage is virtually unavoidable – where the major endeavour is to minimise it to acceptable levels. Also in split-brain rabbits, rats, and mice, it is essential to use a surgical control group where the callosum had been exposed but not sectioned.
3.1.3 Chiasma Bisection and the Lateral Visual Fields
With the possible exception of cetaceans, there is a remarkably consistent organisation in the decussation of the retinal ganglion cell projections in the chiasma. The lateral hemiretinae are the source of the ipsilateral retinal projections, which consist predominantly of cortical binocular cells. The nasal hemiretinae give rise to the contralateral projections, which are exclusively cortical monocular cells. The ipsilateral projections process pattern information from the binocular part of the visual field; whereas the contralateral fibres respond to movement signals from the lateral monocular crescent of the visual field (see a discussion of this issue by Russell et al. (63)). The contralateral system also provides a critical pathway via the nucleus of the optic tract to the cerebellar oculomotor control centres. This retino-cerebellar system controls optokinetic nystagmus and the vestibulo-ocular reflex, as well as conditioned movements of the nictitating membrane response in Pavlovian conditioning (62).
3.1.4 Brain Rigidity
A fourth consideration is the major change that occurs in the rigidity of the brain tissue as a function of phylogeny. The brains of animals, such as the rat or mouse, have very little structural rigidity or firmness. Hence direct mechanical manipulation of the brain using retractors is hazardous. The retractor blades can penetrate into the wall of the midline cortex. In addition, the blood vessels are very fragile, such that moving them to access a commissure can be difficult. In the rabbit, the brain tissue is more resilient than in mouse or rat. It can, however, be “hardened” by the use of i.v. injection of mannitol or albumen. Unfortunately, this technique is not effective in either rats or mice.
4 Surgical Techniques of Midline Sensory Disconnection
In this section, a detailed description is given of the optimal methods for section of the corpus callosum, the anterior and posterior commissures, as well as for chiasma section. In each case, the methodology described is one which we have either modified or developed over the last 30 years to produce consistent results and minimal collateral damage (Fig. 2). Separate and complete descriptions of these procedures will be given for the monkey, the rabbit, and the rat. This is to enable the reader to focus on the procedural details for the species relevant to their research. Nonetheless, it should always be remembered that great differences exist between organisation of the mammalian visual pathways (see Table 1). Unless otherwise stated, it should be assumed that these procedures should be carried out using a full sterilisation procedure for the animal as well as the surgeon.
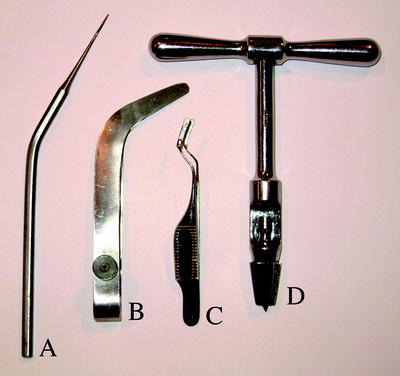

Fig. 2.
Specialised surgical instruments for commissurotomy procedures. (a) A microknife curved to form a linear extension of the hand. It has a cutting blade on the lower edge with a bevelled back on the obverse. side (b) A large speculum/hemisphere retractor suitable for monkey and cat procedures. (c) A microspeculum/hemisphere retractor suitable for rabbit procedures. The blades of the retractors are counter sprung in the open position. During surgery they are manually held together during insertion between hemispheres and then gently released to separate the medial walls between hemispheres.
4.1 Animals Possessing a Falx
4.1.1 Section of the Corpus Callosum in Monkey and Cat
A bone flap is created in the left hemisphere to enable the entire left cortical hemisphere to be retracted from the confines of the cranium in order to permit lateral retraction of the falx to access the corpus callosum without compressing the surface vasculature of the retracted hemisphere. As the procedure is lengthy, the bone flap approach is essential to avoid ischæmic cortical damage. It also prevents the boney flap from necrosis.
In both the monkey and the cat, a short-lasting anæsthetic is used to secure the animal in an appropriate head holder, with the head positioned horizontally. Using a set of pædiatric jaw retractors, the trachea is intubated with a small calibre tube. The animal can then be transferred to gaseous anæsthesia for the remainder of the procedure. It is essential to observe a stringent sterile procedure throughout the callosal section procedure to avoid any infection in the deep regions of midline structures of the brain. The eyes are covered with vasoline to protect the cornea from either dehydration or the effects of sterilisation of the scalp. The entire dorsal surface of the head is first closely shaved with a razor, washed with surgical soap followed by a Zephiran sterilisation of the dorsal surface of the head for 15 min.
The head is then draped with a sterile covering creating a window that exposes the operative area only throughout the operation. An incision is made in the right side of the skull approximately 1 cm over the central suture parallel to the midline, extending from the anterior to the posterior pole of the cranium. From either end of this longitudinal incision, two lateral incisions are added extending down the lateral aspect of the dorsotemporal aspect of the left side of skull to expose the temporalis muscle, which is left attached to the skull. The periosteum is blunt dissected back to each side of the temporalis muscle. In order to shrink the brain and reduce the tension on the blood vessels, 50 ml of 20% mannitol, using a microdrive, are injected i.v. at a rate of 10 ml per min. Throughout this transfusion period, care is taken to maintain the temperature of the mannitol blood temperature, using a water bath with a temperature feedback control from the mannitol bag.
A circular shaped bone segment, starting 0.5 cm in the right hemisphere crossing over into the left hemisphere, is then freed but it remains attached to the temporalis muscle. The bone flap is made by first creating a perimeter of six 5 mm diameter trephine holes as illustrated in Fig. 3. A Gigli saw was threaded via a surgical guide between the trephine holes and the inter-trephine hole. The bone bridges are cut to free the bone flap, with the exception of the part underlying the temporalis muscle. The bone bridges between trephine holes in the right hemisphere are chamfer cut at 45° to provide a support ledge when the flap is replaced.
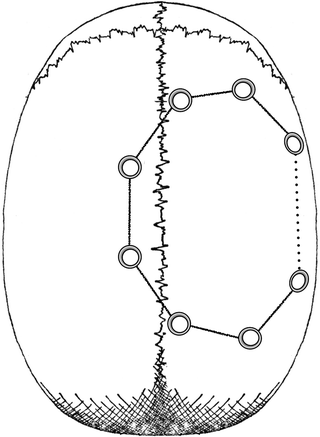

Fig. 3.
Bone flap schematic. Showing a circular array of trephine holes that are connected by use of a Gigli saw to complete the bone flap which remains attached to the temporalis muscle (see text for further details).
Following this, a 3-mm wide flat spatula is inserted between the trephine holes over right hemisphere to drill 0.5 mm diameter holes on either side the chamfered cut. This enables the bone flap to be secured in place, using silver sutures at the end of the procedure. The flap is cautiously elevated at the midline crest, using a dental elevator and small curette to separate the flap from any dural adhesions. Typically these are greatest at the midline region above the central sinus. The bridge between trephine holes beneath the temporalis muscle is separated by elevating the flap until it breaks from the calvarium remaining attached to the temporalis muscle. The bone flap is then wrapped in gauze soaked in Ringer’s solution, and draped laterally away from the hemisphere. To retract the dura mater, a dural hook is used to snare the dura of the left hemisphere, 1 cm lateral to the central sinus. The dura is then elevated and opened by a fine pair of scissors, and a slit parallel to the central sinus is extended from the frontal to the occipital pole of the brain. The dural incision is then extended towards the central sinus at both ends to allow the dural flap to be reflected over the midline as a covering to retract the central sinus against the medial wall of the non-retracted hemisphere. Care is taken to avoid extending the dural section into the sagittal sinus. On occasion there are adhesions between the dura and communicating veins attached to the sinus. No attempt is made to separate or ligate the vessels. A slit is made in the dura on each side of the vein, which enables the dura to be turned back as a discontinuous fold. Cottonoid strips, soaked in Ringer’s solution, are placed over both the exposed dura and cortex.
The number of communicating veins from the cortex into the sagittal sinus are sparse, being rarely more that one or two. Accordingly, it is possible to preserve them by working around them. Ligation of large veins, which on occasion is done, can result in œdema and damage to the exposed hemisphere. Using a speculum and a small gauge aspirator (with a finger controlled pressure release valve) are cautiously inserted between hemispheres. In either the monkey or the cat, there are rarely adhesions between the medial wall and the retracted hemisphere, or the falx in the posterior callosal region. Frequently, there is a substantial arterial plexus over both the splenium and the body of the callosum dorsal to the anterior massa intermedia. Care is essential to avoid damage to these arteries. With the surgical microscope, a dental elevator is used to manipulate cottonoid balls soaked in sterile Ringers which in turn manipulate these vessels and to enable access to the callosum. This is sectioned using the speculum with one hand and alternating the sucker and angled knife with the other hand. With a mixture of care and caution, the section can be accomplished with little or no damage to the medial walls of the hemispheres. Starting the transection at the splenial end of the callosum, csf from the underlying third ventricle will flow into the section area, which is easily controlled by low pressure suction. As the section continues rostrally, the massa intermedia can be seen as a distinct grey structure in contrast to the bright white of the corpus callosum. The arterial plexus at the anterior rim of the mass intermedia is a critical landmark that indicates the sectioning procedure is on the midline.
Following the completion of the transection and staunching any blood seepage from fine vessels, the cavity is irrigated with Ringers at blood temperature to see if there is any further bleeding. Only when it is certain that there is no vascular leakage, are the hemispheres re-opposed. Before replacing the bone flap, the entire exposed surface of the exposed hemispheres is carefully covered with a pre-shaped gel film cover, which is tucked under the margins of the bone defect. Then the bone flap is secured in place by silver sutures, holding the chamfered edges of the bone flap together. The margins of the periosteum are sutured across the bone defect, as are the margins of the scalp, which are closed with discontinuous mattress sutures, to prevent any damage due to any scratching of the incision by the animal after recovering consciousness. Before the anæsthesia is turned off, 30 cc of body temperature sterile isotonic saline is given i.v. to protect against any dehydration. Cortisone treatment is also given to prevent post operative brain œdema in combination with appropriate pain medication. Both treatments are continued for the next 5 days. On the first post-operative day, the animal should be alert and without any motor deficits.
4.1.2 Section of the Optic Chiasma in Monkey and Cat
Section of the optic chiasma and the anterior commissure can be achieved via an extension of the dorsal approach to the corpus callosum first pioneered by Dandy (2). The disadvantage of this procedure in neither monkey or cat it is neither a necessary nor an optimal approach in these animals. Another disadvantage of the approach is that access to the optic chiasma is of necessity by threading through the circle of Willis (which is both hazardous and extremely difficult). Downer (31), adapted the transbuccal procedure of Cushing for accessing dissecting tumours of the chiasma, to the macaque and the cat, to section the optic chiasma. This approach has advantages over the dorsal route, in that the entire chiasma is visible via the surgical microscope. It avoids the circle of Willis, and does not involve the heroic passage between hemispheres from cortex to the ventral surface of the brain.
Using a short-lasting anæsthetic, the animal is intubated with a small calibre tube, and then transferred to gaseous anæsthesia. The monkey is placed in a supine position with the head dorsiflexed and secured in a head holder. The eyes are covered with vaseline, and then covered with surgical tape to prevent any corneal dehydration. The mouth is held open with a pædiatric jaw retractor. Using full aseptic procedure, the field around the mouth is covered with sterile drapes. The mouth is then thoroughly washed with a solution of isotonic saline followed by Zephiran for 15 min. A “U”- shaped incision is made through the soft palate through the nasopharynx. The incision begins laterally and caudally just anterior to the isthmus of the fauces, and is extended rostrally to approximately 2 mm over the caudal margin of the bony palate, and then is curved around to the other side and returned to the same point where it was begun. The underlying membraneous septum is severed, and the flap thus formed is reflected from the field. The spinous process of the vomer is removed with a small pair of rongeurs. With a small curette the mucosa overlying the vomer and sphenoid bones is also removed. The entire vomer and buccal cavity are filled with Zephiran (1:5,000) for a further 15 min.
From this stage onwards, all instruments used in the surgical field are placed in 70% alcohol on being removed from the field. They are only returned to the incision via a sterile saline rinse in an attempt to keep the field sterile. A dental burr (with a long shank and large spherical cutter) is centred on the suture separating the sphenoid bone from the vomer, and a hole drilled through into the cranial cavity. The hole is then enlarged anteriorly in the midline until the optic nerves can be seen joining to form the optic chiasma, and posteriorly until the anterior hypophysis is clearly visible. Throughout this approach, sterile bone wax is applied into bone trabiculae of the cavity to control bone bleeding. Using a Zeiss long focus surgical microscope, the optic chiasma is cut longitudinally in the midline (Fig. 4). A preshaped rectangle of gel film is placed over the chiasma, carefully tucking the sides of the film beneath each side of the rectangular boney defect. A fast curing liquid bone cement is then applied with a dental spatula to seal the cavity. This will set within a few minutes to form a hard, bone-like plug, sealing the cavity. The edges of the palatal flap are then closed with interrupted ophthalmic silk sutures, as well as a continuous plain cat-gut suture. Post operatively the monkey is given antibiotics and analgesics daily for 10 days.
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


