Fig. 1.
An example template for scoring motor behaviour. Although this describes the bridge test, similar parameters apply to all the fundamental tests and the rotarod. T1–T3 indicate the trial number on each testing day. Qualitative data such as sidedness of movement or type of gait will need to be given a numerical score for subsequent inter-group comparisons (5, 10). These can be identified on the templates.
2.1 General Motor Function
General tests of cerebellar motor control are performed three times a day for 5 days. We find that if they are continued for longer, the animals clearly become bored and performance decreases. For each test, there is an upper time limit of 3 min. This value was calculated from the performance of normal animals, which is then multiplied threefold to allow adequate time for an ataxic animal to attempt the test without confounding performance through becoming fatigued (10); see Note 1. For each animal on each day, the data for the three tests are averaged. This involves giving a numeral to qualitative data such as sidedness (Fig. 1).
2.1.1 Dynamic Postural Reflexes
The cerebellum and its vestibular circuitry play key roles in dynamic postural adjustment. Postural reflexes are quickly and easily tested but form only a relatively crude index of cerebellar dysfunction and thus are not always discriminative for minor lesions. They are particularly useful in unilateral cerebellar lesions and those undertaken during development. The two main tests are the righting reflex and vestibular drop. Neither of these tests requires special equipment although, to reduce stress levels, it is better not to have the room lighting too bright.
1.
Righting reflex. Animals are momentarily held supine by the shoulders and hip-girdle on a flat surface and released. Normal animals will immediately turn over to recover their normal prone quadruped stance. The presence/absence of the reflex, time taken and direction of response are noted (see Note 2).
2.
Vestibular drop. When an animal is suspended by the tail it arches its body to bring its head up to the level of its tail. This procedure also tests truncal muscular strength as well as vestibular function. The presence/absence of the reflex, denoted success/failure, within 60 s (the short upper time limit is to minimize distress), time and direction taken are observed (see Note 3a–c).
2.1.2 Quadruped Locomotion
The patterns of simple gait can be tested through a bridge-crossing task and footprint analysis.
1.
Foot print analysis.
This test is very simple and it often used as a general indicator of motor function, but it also provides useful information about cerebellar ataxia.
Equipment. While this test can be done on an open surface without specialist equipment, it is easier to create a narrow passage which is well lit at the start and has a dark escape box at the other end. We used an alley 19 cm wide and 80 cm long for adult rats, which is also suitable for mice (see Note 4a).
Method. Footprints are made using waterproof ink to mark the paws and are revealed on absorbent white paper lining the passage.
1.
2.
Animals are placed in the corridor facing away from the tester toward the dark escape box.
3.
The animals are enticed to walk forward simply by turning on a light at the “start” end of the corridor (see Note 4c).
Analysis. Regularity of footprints, their overlap in normal animals, stride length (left–left and right–right), stride width (left–right) and the angle of external rotation of the hind foot are measured (10, 13). Rotation angle is measured from the back of the heel to the centre of the third toe and it is increased in ataxic animals (Fig. 2; see Note 4d).
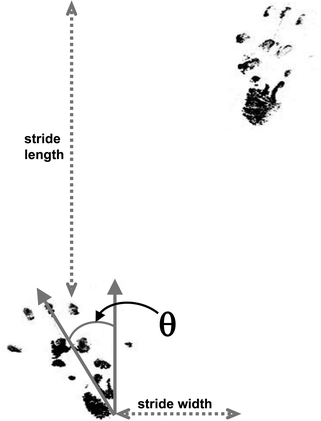

Fig. 2.
An example of rat hindpaw prints showing the measures that can be taken. Although stride length can be measured as shown from one footprint (left) to the next (right); it is more useful for animals with unilateral cerebellar lesions to measure the length between foot-strikes of the same side, as these can be asymmetric. θ = angle of rotation between the axis of the foot and the direction of walking.
2.
Crossing a narrow bridge.
Crossing a narrow bridge is another general motor test which can provide information about cerebellar motor control.
Equipment. The bridge needs to be at least 60 cm above a foam padded base (to prevent injury in the event of a fall) and 60 cm long (Fig. 3). The addition of distance markers along the side facilitates data acquisition. We use a wooden bridge with a square profile, which makes the bridge width important; if it is too narrow, ataxic animals cannot cross it without falling and therefore tend to freeze soon after they start (see Note 5a). For adult rats with unilateral cerebellar lesions, 3 cm width is a difficult and discriminatory task (5, 10), but mice require <2 cm width.
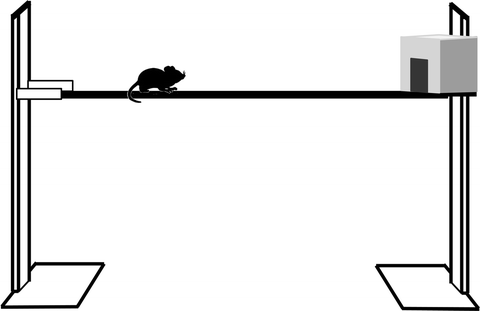

Fig. 3.
Schema showing a narrow bridge to test locomotor function. It is 60 cm long and 60 cm above the base but width will vary depending on species.
Method. Animals are placed on one end of a bridge and have to progress to a box on the other end (see Note 5b).
2.1.3 Complex Locomotor Skill
The complex coordination and muscular strength of the animals also provide useful insights into cerebellar function. This can be tested by climbing a ladder and the ability to progress along a wire.
1.
Ascending a ladder.
Climbing a ladder can also be used to assess coordinate gait as it demands repeated even stepping to place each foot on the subsequent rung. While it provides only a relatively crude screen of cerebellar ability, it is useful to assess maturation of motor function in animals lesioned during development. It is not sufficiently discriminatory in animals with unilateral cerebellar lesions
Equipment. A steel ladder 8 cm wide comprises 18 rungs, each 5 mm in diameter and 2 cm apart rising at an angle of 25° to the vertical to a dark covered platform (Fig. 4). The whole ladder is placed on the edge of a bench/table so that from its base there is a drop to the floor. This is necessary to induce the animal to climb.
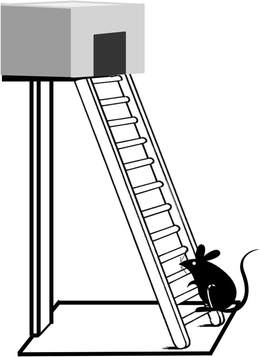

Fig. 4.
Schema showing a ladder for assessing locomotor function. It has 18 rungs, each 5 mm in diameter and 2 cm apart. It rises at an angle of 25° to the vertical to a dark covered platform.
Method. Animals are placed on the bottom rung so that they climb to the top (see Note 6).
Analysis. Successful arrivals at the top platform, time on ladder and number of rungs climbed, plus number of hindfoot slips are recorded. Time spent in pauses or freezing is subtracted from the total time taken.
2.
Progression along a wire.
Although hanging from a wire (or coat hanger) is generally used as a test of muscular strength, a horizontal wire provides a test of trunk and limb muscle coordination. Animals will use their tail and hind limbs to progress along a wire to a box at one end to escape from falling (10, 14).
Equipment. The apparatus consists of a horizontal wire suspended between upright supports on which there are escape platforms. The wire is 3 mm in diameter, 70 cm long and 60 cm above a foam base.
Method. Animals are suspended by their front paws from the middle of the wire (see Note 7a) and are required to climb along the wire to a box at either end.
Analysis. The ability of the animals to reach and enter the box, the distance traversed, the direction taken, time taken, falls and the use of supporting hind limbs are recorded (see Note 7b).
2.2 Motor Synchronisation Task
A key cerebellar function, which directly correlates to human cerebellar dysfunction, is the synchronisation of repetitive movements (e.g., walking or running 15). The rotarod is a sensitive measure of this task as it is able to detect even minor abnormalities of gait synchronisation (13, 15–17). Moreover, it is also simple enough for animals with highly disordered cerebellar cortex or unilateral cerebellar lesions to still perform the task at slow speeds (10, 18, 19) and therefore allow lesion/treatment comparisons (see Note 8).
Equipment. The rotarod is a horizontal cylinder, which can either be made “in house” or is commercially available for mice, rats or combined (e.g., Panlab/Harvard Apparatus Rota Rod LE8500). Commercially available apparatuses have automatic timers to measure when the animal falls; whereas this has to be manually timed for “in house” machines (see Note 8a). The rod is mounted 50 cm above a foam base. It is 30–50 cm long, covered in adhesive plaster to allow grip and divided into lanes for each animal. Cardboard/plastic circles make a useful option for adjusting the number and size of lanes. The cylinder rotates about its long axis at either constant or accelerating speeds.
Method. The machine is set to rotate at either constant or accelerating speed.
1.
The constant speed chosen varies with the experimental paradigm/degree of ataxia, but increments of 10 rpm starting from 10 rpm provide a discriminatory range for cerebellar testing.
2.
Accelerating tests start at 4 rpm and increase by 4 rpm every 30 s over 5 min to a maximum of 40 rpm (see Note 8b).
3.
Irrespective of the test, the animal is placed on the rotating cylinder perpendicular to the direction of rotation facing away from the tester. This is best achieved by allowing the animals to walk off the open palm onto the rotating rod. In order to maintain position on top of the rod and not fall off, it has to walk forwards synchronising stepping frequency and stride length to the speed of rotation. For each trial, the parameters recorded are:
(a)
Total time on the rod.
(b)
Time walking and time spent in error (clinging or walking backwards).
(c)
Time to first error (fall or cling).
The trial ends either when the animal falls or 180 s is reached (but see Note 8c).
Analysis. For each animal on each day the mean walking latency-to-error (fall or cling) and/or total walking time (total time – time spent clinging/reverse walking) is calculated. The mean time on the final day at each speed is used to compare the performance of the experimental groups (see Note 8e).
2.3 Motor Learning
In addition to coordination and synchronisation of repetitive movements, cerebellar control of fine movement also involves motor learning (10, 15, 20, 21). The type of motor learning described here is not classical conditioning reflexes such as the eyeblink reflex (e.g., review 22), but rather the learning of motor patterns required for complex movements. The capacity to evaluate this more cognitive aspect of cerebellar motor control becomes crucial to detect differences between groups which may be missed by less discerning tests and to differentiate the function of different regions of the cerebellum, specifically its cortical circuit.
2.3.1 Learning Motor Synchronisation
As the cerebellar cortex regulates gait synchronisation and motor learning, differences between experimental groups may result from motor skills deficit due to the lesion/pathology, an inability to learn the task or a combination of both. These different (dys)functions can be evaluated using data from the rotarod.
Equipment and method. See constant speed testing of gait synchronisation above (Sect. 2.2).
Analysis. Discrimination between deficits in motor performance and learning can be assessed by comparing the differences in error-latency (relative motor deficit) in untrained animals (first day of testing), when any between-group differences will be due only to motor impairment, with trained animals whose performances will reflect both motor and learning ability (10, 15); Fig. 5). The relative motor deficit is calculated as (control group time − experimental group time)/control time; 10, 15). The relative motor deficit in animals that can learn a task will remain similar or decrease (a to b in Fig. 5), whereas in animals which are unable to learn the task, the relative motor deficit to the control group remains almost unchanged or increases (Fig. 5 points “c” to “d”; see Note 9).
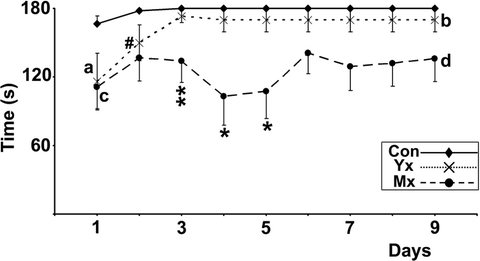

Fig. 5.
Learning impairment calculation from data obtained from a 10-rpm constant speed rotarod. Control animals learn to walk for the maximal time without error within the first training day. Although young lesioned animals (Yx) animals learn more slowly over 3 days, they improve their relative deficit to controls from the start of training (“a”) to the end of training (“b”), which demonstrates learning. In contrast, mature lesioned animals (Mx) do not improve error-latency or relative impairment (compare “c” to “d”) with training, i.e. they did not learn. Consequently, the increased relative deficit between Mx and Yx animals (“a”–“c” vs. “b”–“d”) reveals an inability to learn gait synchronisation in the Mx group. Significant differences between Yx and control; #p < 0.05: significant differences between Mx and control; *p < 0.05, **p < 0.01. Reprinted from (10), Copyright (2005), with permission from Elsevier.
2.3.2 Learning Motor Patterns
In addition, the cerebellum is also involved in spatial navigation (21, 23–26), specifically its role to learn the motor patterns required to make a direct path to a specific target and link them to inputs from the environment (21, 27). Since cerebellar lesions have less effect on swimming than on terrestrial locomotion (9), comparing swimming ability to visible vs. hidden platforms in a Morris water maze (28) adds further evaluation of cerebellar motor learning (but see Note 10).
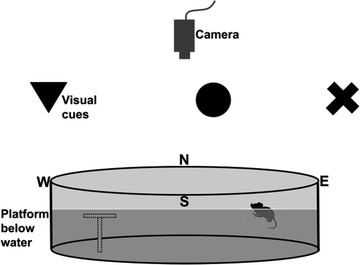
Equipment. The water maze is a circular pool (120 cm in diameter for rats, 90 cm for mice) filled with water (21°C; see Note 10a) and a clear 15-cm diameter Plexiglas escape platform is positioned in one quadrant (see Note 10b). The maze is located in a room with numerous extra-maze cues (24, 29–32), white noise and a video-tracking camera above (Fig. 6; see Note 10b).
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


