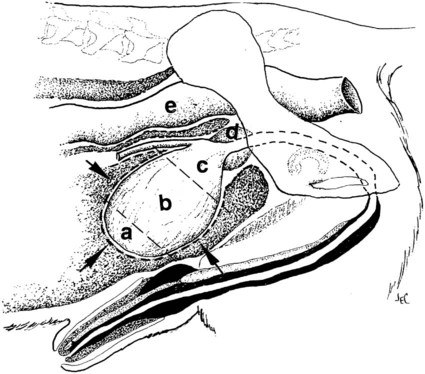
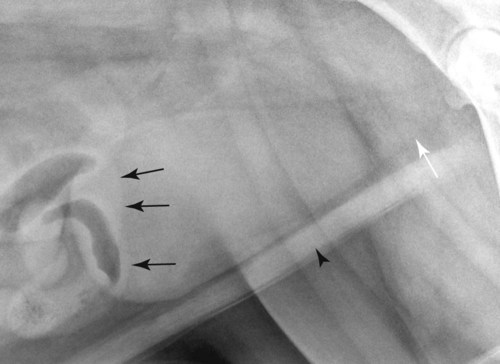
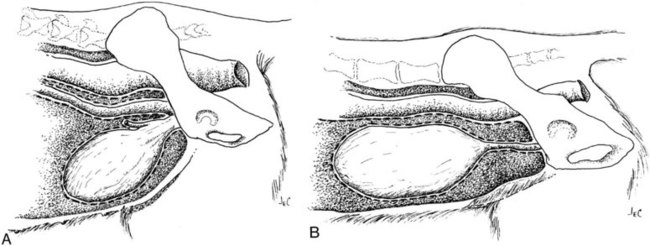
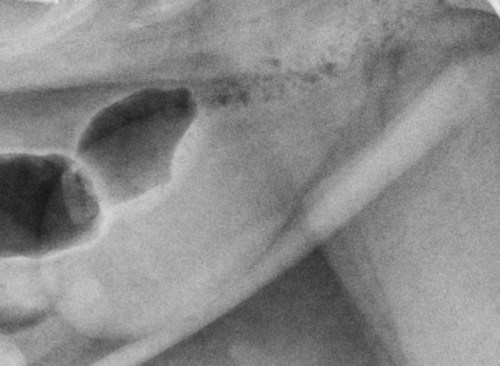
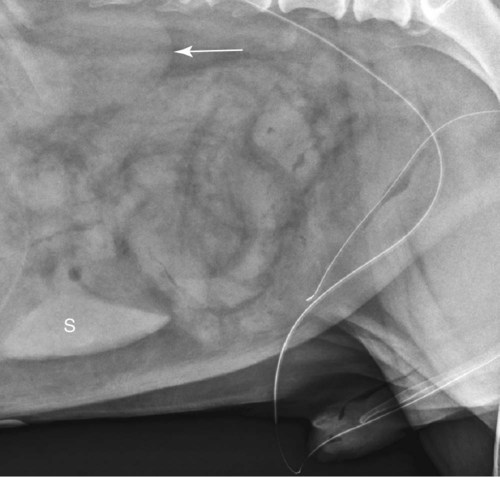
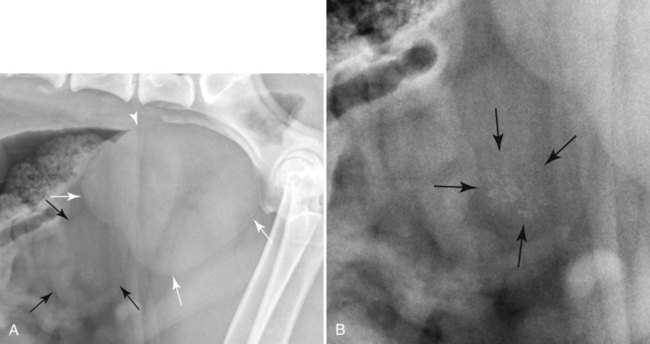
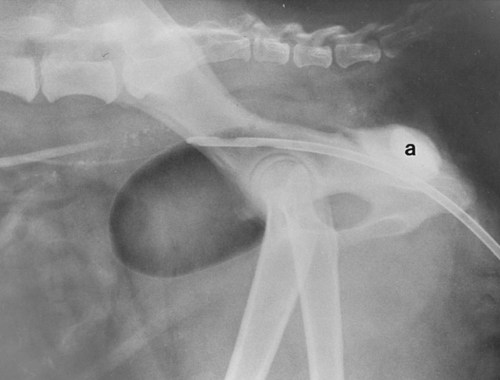
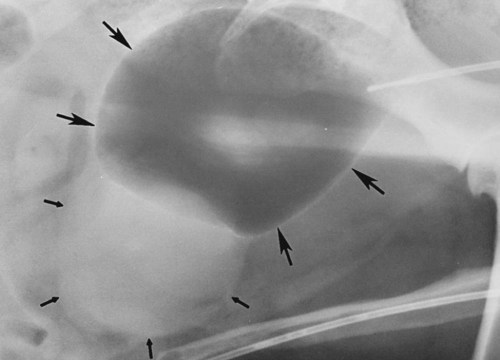
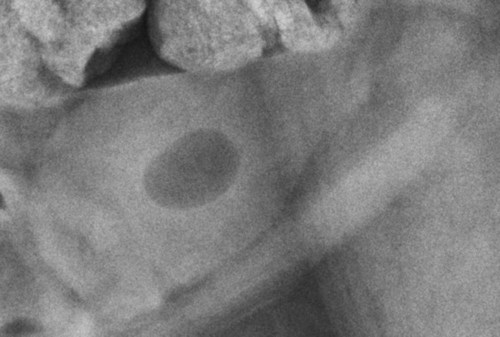
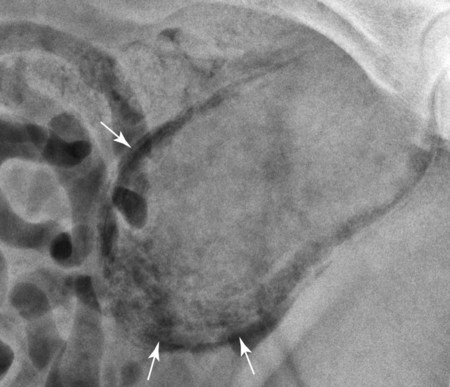
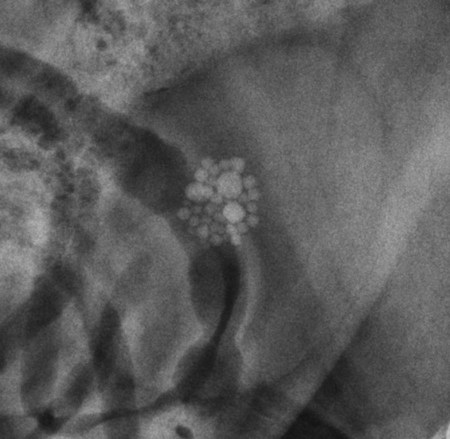
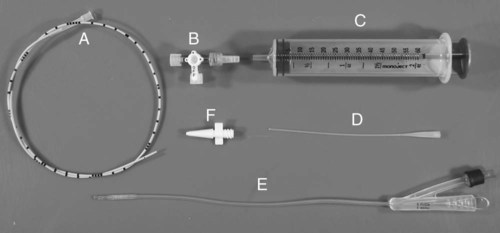
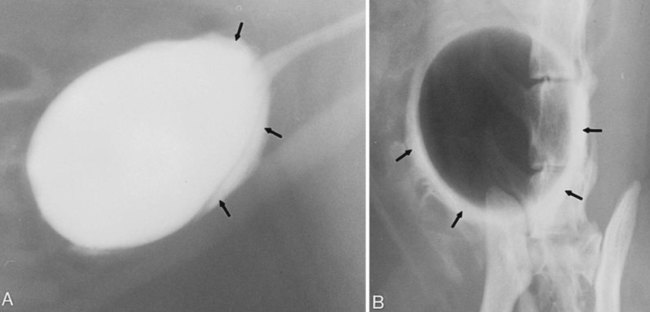
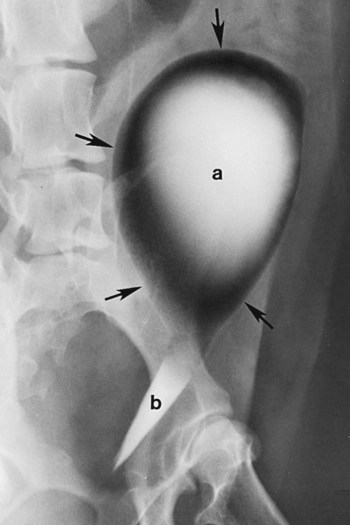
The urinary bladder is divided grossly into three parts: the apex or vertex (apex vesicae) cranially, the body (corpus vesicae) in the middle, and the neck (cervix vesicae) caudally (Fig. 39-1).1,2 Three ligaments formed from peritoneal reflections hold the bladder loosely in position.2 The middle bladder ligament (ligamentum vesicae medianum) extends along the ventral bladder surface, and two lateral ligaments (ligamenta vesicae lateralia) extend along the lateral bladder surfaces. These ligaments often contain large fat deposits, facilitating radiographic visualization of the bladder neck and body. The cranial and dorsal surfaces of the bladder are visible radiographically because of adjacent fat within the omentum and mesentery (Fig. 39-2). Bladder size varies with the amount of urine. The bladder is small after voiding and may not be visible radiographically. With extreme distention, the apex/vertex may extend to the umbilicus. Severe distention may occur normally if the animal has not voided owing to lack of opportunity or because of a strange or unfamiliar environment. The urinary bladder in the dog is usually oval, but with distention it becomes more ellipsoid. The feline urinary bladder is almost always ellipsoid (Fig. 39-3). The bladder is cranial to the pubis, dorsal to the rectus abdominis muscle, caudal to the small bowel and omentum, and ventral to the large bowel. In females, the caudal aspect of the uterus lies between the bladder and colon/rectum. The caudal portion of the normal urinary bladder may be cranial to the pubis or within the pelvic canal.3,4 When distended, the caudal part of the urinary bladder is usually cranial to the pubis.3,4 The normal urinary bladder in the cat is always intraabdominal, being located 2 to 3 cm cranial to the pubis because of the long bladder neck in this species (Fig. 39-4).5 Survey radiographic signs of urinary bladder disease are limited. In many instances, abnormalities indicate disease in adjacent structures. Signs that indicate disease of the urinary bladder or adjacent structures are poor or nonexistent bladder visualization and abnormal bladder position, shape, size, and opacity (Table 39-1). Table • 39-1 Urinary Bladder: Survey Radiographic Signs Poor radiographic visualization of the urinary bladder may occur regardless of whether serosal detail in the caudal abdomen is good or decreased. If serosal detail is good and the bladder is not seen, the bladder is empty or has been displaced caudally or ventrally. If serosal detail is decreased and the bladder surface is not seen distinctly, free peritoneal fluid or inadequate peritoneal fat may be the cause (Fig. 39-5). The bladder may be displaced abnormally in various directions.6 The cause of bladder displacement may sometimes be determined by observation of surrounding structures (Fig. 39-6). With severe bladder displacement, as with a hernia, the bladder may not even be visible radiographically but can be identified using cystography or ultrasonography. Bladder retroflexion was found in 12 (20%) of 61 dogs with a perineal hernia.7 A urinary bladder partially within the pelvic canal (Fig. 39-7) may be associated with congenital urinary tract anomalies.8 A minimally distended bladder may be pelvic in position normally and move more cranially when distended; a pelvic bladder has been reported as a normal variation.4,9 Incontinent female dogs with a pelvic bladder usually have a shorter urethra than continent dogs.10,11 Because a pelvic bladder does not always indicate a clinical problem, a pelvic bladder should be correlated with clinical signs to determine its clinical significance. A change in shape of the urinary bladder is not detected commonly. Abdominal masses adjacent to the serosal surface of the bladder may distort the bladder shape. Tumors originating from the bladder wall occasionally protrude from the serosal surface and produce a discernible bladder shape change (Fig. 39-8).12 A pointed apex with an elongated bladder may occur with a persistent urachal ligament.13 An abnormally small or large urinary bladder is difficult to diagnose radiographically because of the wide variation in normal bladder size. In most instances, a consistently small or large bladder with associated clinical signs is an indication that a contrast study or ultrasound examination should be performed to determine the cause of the suspected size change. Any change in radiographic opacity in the urinary bladder is abnormal and usually easy to detect. Gas in the bladder may be introduced iatrogenically from catheterization or cystocentesis. Small luminal gas bubbles are usually seen in the center of the bladder on a recumbent lateral view (Fig. 39-9). Gas in the bladder lumen, bladder wall, and occasionally the bladder ligaments occurs with emphysematous cystitis (Fig. 39-10). Emphysematous cystitis is produced by glucose-fermenting organisms and may be seen in association with diabetes mellitus.14 Occurrence of emphysematous cystitis without diabetes mellitus also has been reported.15,16 Most radiopacities associated with the bladder are calculi (Fig. 39-6, B; Fig. 39-11). Opacities cannot be confirmed to be in the bladder based on one radiograph; confirmation requires examination of orthogonal radiographs. If doubt exists regarding the location or presence of cystic calculi after examining orthogonal radiographs, sonography would be the most expeditious way to obtain further information. If cystic calculi are identified, the remainder of the urinary tract from the kidneys to the terminus of the urethra should be examined for additional calculi. Not all calculi are radiopaque; thus the absence of radiopacities within the bladder does not rule out the presence of cystic calculi (Table 39-2). In cats with suspected feline idiopathic cystitis, survey radiographs and cystography can be used to evaluate the bladder. Often, mild bladder wall thickening may be observed with cystography.17 Additionally, a horizontal-beam radiograph can be made to diagnose sandlike material that will collect within the dependent portion of the bladder. This additional view may be helpful in cats with feline idiopathic cystitis (feline urologic syndrome).18 Radiopacities in the urinary bladder can also be produced by mineralization of the bladder wall associated with neoplasia or chronic cystitis, but this is unusual.19 Table • 39-2 Radiopacity of Cystic Calculi on Survey Abdominal Radiographs Voiding cystography is not discussed in this chapter. The reader is referred to other publications for more information on this technique.20–23 Voiding cystography coupled with cystometry and urethral pressure profiles is the technique of choice for investigating dynamic bladder disease such as urinary incontinence and urethral incompetence.24 All catheters and equipment should be sterilized, and the genitalia should be cleaned before the bladder is catheterized. The equipment necessary for bladder catheterization is illustrated in Figure 39-12. To reduce bladder pain and spasm during cystography, 2 to 5 mL of 2% lidocaine (Xylocaine) without epinephrine may be injected into the bladder before cystography is performed. Complications resulting from catheterization and cystographic procedures occur infrequently and are usually not detrimental to the animal. Iatrogenic trauma, bacterial contamination,25 or kinked26 and knotted urethral catheters may occur from improper catheterization techniques. Intramural and subserosal accumulation of contrast medium has been reported after maximal bladder distention with a Foley catheter (Fig. 39-13).27–30 This complication occurs more frequently in cats, often with a nondistended bladder and minimal intravesicular pressure. It usually does not result in a clinical problem. Mucosal ulceration, inflammation, and granulomatous reactions may occur, but the changes are usually transitory and produce no serious clinical problems.9 The most serious complication from negative-contrast cystography is gas embolization into the circulatory system, which may result in death.31–33 Fortunately, such complications occur rarely. Death from gas embolism may be prevented by using more soluble nitrous oxide or carbon dioxide instead of room air. Both negative- and positive-contrast media are used for cystography. Negative-contrast media include room air, carbon dioxide, and nitrous oxide. Positive-contrast media are water-soluble organic iodides that should be used in an approximate 20% iodine solution. Barium should never be used for cystography.27,34 The volume of positive-contrast medium used for cystography varies with body weight, the species, and the pathologic process present in the bladder. An approximation of 10 mL, or a range of 3.5 to 13.1 mL of contrast medium per kilogram body weight, may be used.34 The injection should be terminated before the estimated volume has been administered if the bladder feels adequately distended by external palpation, if reflux occurs around the catheter, or if back pressure is felt on the syringe plunger. Moderate bladder distention is recommended because maximal distention may obliterate subtle mucosal and bladder wall changes.35 Four radiographic views of the caudal abdomen (lateral, ventrodorsal, ventral left–dorsal right, ventral right–dorsal left) should be made to examine the contrast-medium–filled bladder adequately. A double-contrast cystogram is performed by injecting a small volume of undiluted positive-contrast medium into an empty bladder. The recommended dose of positive-contrast medium is 0.5 to 1 mL for a cat, 1 to 3 mL for a dog weighing less than 25 lb, and 3 to 6 mL for animals weighing more than 25 lb. Positive–contrast-medium injection is followed by bladder distention with negative-contrast medium (Fig. 39-14). Double-contrast cystography is better than positive-contrast cystography for assessing bladder wall lesions and intraluminal filling defects. The selection of positive- or double-contrast cystography is based on clinical history, clinical signs, radiographic signs, and the character of aspirate obtained with bladder catheterization (Table 39-3). Table • 39-3 Indications for Type of Cystographic Procedure
The Urinary Bladder
Normal Anatomy
Radiographic Signs of Urinary Bladder Disease
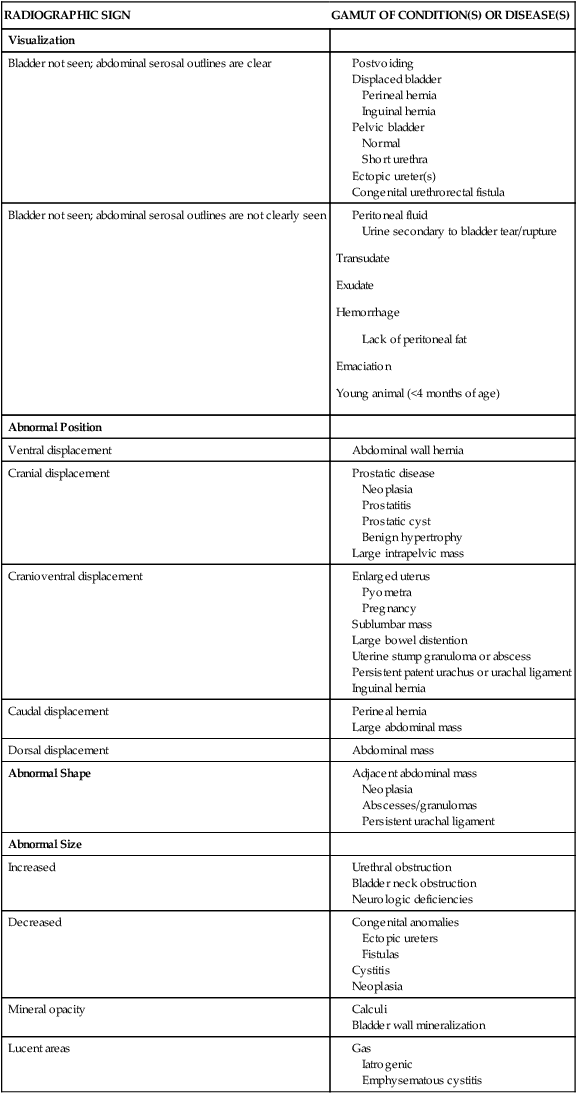
RADIOGRAPHIC SIGN
GAMUT OF CONDITION(S) OR DISEASE(S)
Visualization
Bladder not seen; abdominal serosal outlines are clear
Bladder not seen; abdominal serosal outlines are not clearly seen
Abnormal Position
Ventral displacement
Cranial displacement
Cranioventral displacement
Caudal displacement
Dorsal displacement
Abnormal Shape
Abnormal Size
Increased
Decreased
Mineral opacity
Lucent areas
CALCULUS COMPOSITION
OPACITY
SURFACE CHARACTERISTICS
Calcium oxalate
Moderately to markedly radiopaque
Sharp projections or smooth
Calcium phosphate
Moderately to markedly radiopaque
Smooth
Struvite (ammonium phosphate)
Moderately to markedly radiopaque
Usually smooth; can be spiculated
Silica
Moderately radiopaque
Jackstone appearance
Urate
Nonradiopaque to faintly radiopaque
Smooth
Cystine
Nonradiopaque to faintly radiopaque
Smooth
Contrast Cystography
Cystography Technique
Cystographic Procedures
CONTRAST STUDY
INDICATIONS
Double-contrast cystogram
Suspect bladder wall lesion(s), filling defects (calculi, blood clots, masses)
Positive-contrast cystogram
Suspect ruptured or abnormal bladder location
Negative-contrast cystogram
Not preferred; can determine bladder location
The Urinary Bladder