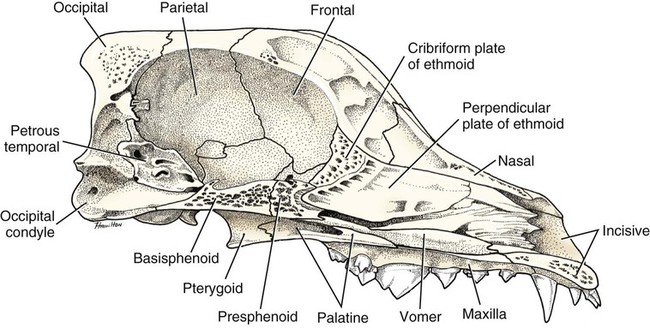
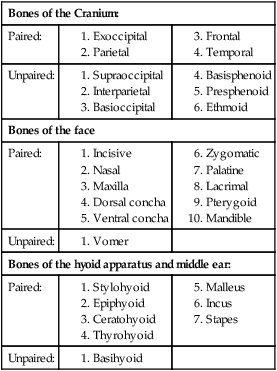
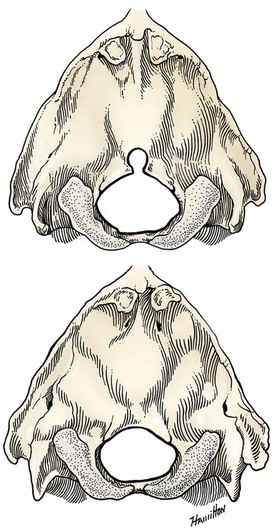
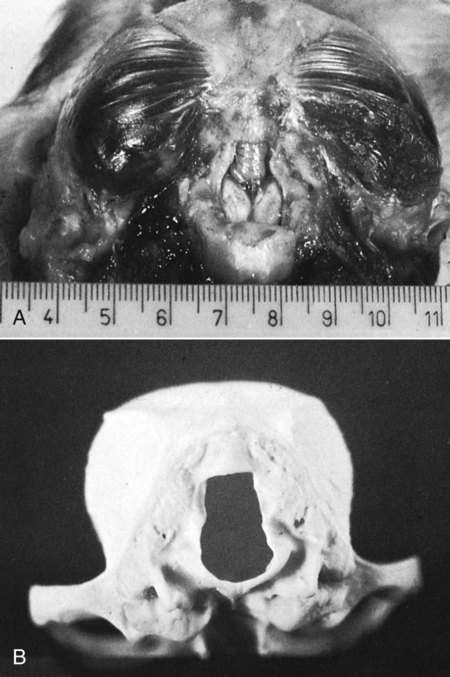
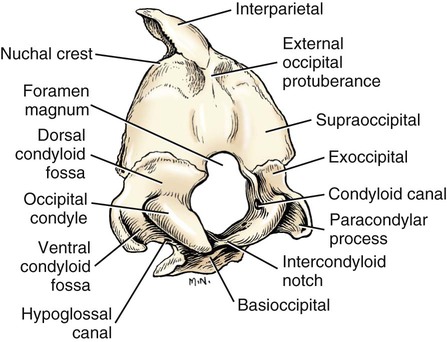
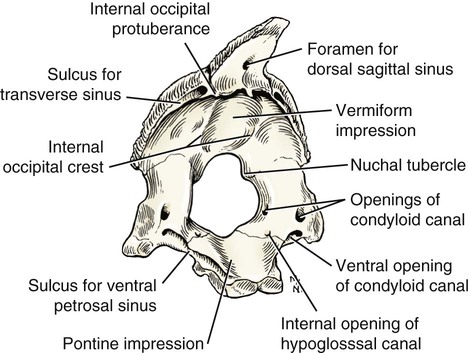
The skeleton serves for support and protection while providing levers for muscular action. It functions as a storehouse for minerals and as a site for fat storage and blood cell formation. In the living body the skeleton is composed of a changing, actively metabolizing tissue that may be altered in shape, size, and position by mechanical or biochemical demands. For a consideration of various aspects of development, maintenance, and repair of the skeleton, reference can be made to Kimmel and Jee (1982), Kincaid and Van Sickle (1983), Jurvelin et al. (1988), and Marks and Popoff (1988). The process of bone repair and the incorporation of heavy metals and rare earths (including radioisotopes) in the adult skeleton attest to its dynamic nature. Bone responds in a variety of ways to vitamin, mineral, and hormone deficiencies or excesses. Inherent in these responses are changes in the physiognomy, construction, and mechanical function of the body. For a review of the history of the vertebrate skeleton and the bones that constitute it, reference may be made to comparative anatomy texts, such as The Vertebrate Body by Romer and Parsons (1986) or Hyman’s Comparative Vertebrate Anatomy by Wake (1979). Much useful information on the skeleton can be found in such older works as Owen (1866), on all vertebrates, and Flower (1870), on mammals. Specific information and references on the skeleton of the dog and other domestic animals can be found in current veterinary anatomy texts and the classic out-of-print Handbuch der Vergleichenden Anatomie der Haustiere by Ellenberger and Baum (1943). For a helpful atlas of radiographic anatomy, see Schebitz and Wilkins (1986). For a discussion of the structure and function of bone in health and disease, reference may be made to The Biochemistry and Physiology of Bone by Bourne (1972, 1976), The Biology of Bone by Hancox (1972), Biological Mineralization by Zipkin (1973), The Physiological and Cellular Basis of Metabolic Bone Disease by Rasmussen and Bordier (1974), and Bone: A Treatise by Hall (1989-1992). For more recent reviews of bone and cartilage biology see Buckwalter et al. (1995), Hall (2005), and Pourquie (2009). Various aspects of skeletal morphology in the dog have been considered by multiple authors: Lumer (1940) has studied evolutionary allometry; Stockard (1941), genetic and endocrine effects; Haag (1948), osteometric analysis of aboriginal dogs; Hildebrand (1954), Clutton-Brock et al. (1976), and Wayne (1984, 1985, 1986), studied comparative skeletal morphology in canids; and Huja and Beck (2007), described bone remodeling of the maxilla, mandible, and femur in young dogs. Bones may be grouped according to shape, structure, function, origin, or position. Heterotopic bones are defined by position and may be located anywhere in the body. The os penis or baculum is an example of such a bone. It is located in the glans of the penis and can be found in all mammals except humans, whales, and some others (Chaine 1926). It functions to stiffen the glans and dilate the fundus of the vagina. Its homolog in the female is the os clitoris, which is more restricted in occurrence and usually absent in the dog (see Chapter 9, Urogenital System). The total average number of bones in each division of the skeletal system, as found in an adult dog (Figs. 4-1 and 4-2), is given in Table 4-1. (Sesamoid bones associated with the limbs are included.) In this enumeration, the bones of the dewclaw (the first digit of the hindpaw) are not included, because this digit is absent in many breeds of dogs, and in other breeds a single or double first digit is required for show purposes (American Kennel Club, 2006). Because dewclaws are nonfunctional, but are frequently injured or ingrown and require treatment, they are often removed. TABLE 4-1 Long bones (ossa longa) are characteristic of the limbs. The bones of the thigh and arm, that is, the femur and humerus, are good examples. Typically a long bone, during its growth, possesses a shaft, or diaphysis, and two ends, the epiphyses (Haines 1942). During development each end is separated from the shaft by a plate of growing cartilage, the physeal cartilage. The epiphysial cartilage (cartilago epiphysialis) is the cartilage on the articular surface of the epiphysis. The rapidly growing, flared end of the bone between the shaft and the epiphysis is called the metaphysis. At maturity the physeal cartilage ceases to grow, and the epiphysis fuses with the shaft as both share in the bony replacement of the physeal cartilage. Farnum and Wilsman (1989) and Farnum et al. (1990) have studied chondrocytes of the growth plate cartilage in situ using differential interference contrast microscopy and time-lapse cinematography. They were able to visualize living hypertrophic chondrocytes as they pass through a sequence of phases, including proliferation, hypertrophy, and death at the chondroosseous junction. Fractures sometimes occur at the physis. Usually after maturity no distinguishable division exists between epiphysis and diaphysis. The ends of most long bones enter into the formation of freely movable joints. Long bones form levers and possess great tensile strength. They are capable of resisting many times the stress to which they are normally subjected. The stress on long bones is both through their long axes, as in standing, and at angles to these axes, as exemplified by the pull of muscles that attach to them. Although bones appear to be rigid and not easily influenced by the soft tissues that surround them, soft tissues actually do contour the bones. Indentations in the form of grooves are produced by blood vessels, nerves, tendons, and ligaments that lie adjacent to them, whereas roughened elevations or depressions are produced by the attachments of tendons and ligaments. The ends of all long bones are enlarged and smooth. In life, these smooth surfaces are covered by a layer of hyaline cartilage, as they enter into the formation of joints. The enlargement of each extremity of a long bone serves a dual purpose. It diminishes the risk of dislocation and provides a large bearing surface for the articulation. The distal end of the terminal phalanx of each digit is an exception to the stated rule. Because it is covered by horn and is not articular, it is neither enlarged nor smooth. Irregular bones (ossa irregulata) are those of the vertebral column, but the term also includes all bones of the skull not of the flat type, and the three parts of the hip bone (os coxae). Jutting processes are the characteristic features of irregular bones. Most of these processes are for muscular and ligamentous attachments; some are for articulation. The vertebrae of quadrupeds protect the spinal cord and furnish a relatively incompressible bony column through which the propelling force generated by the pelvic limbs is transmitted to the trunk. The vertebrae also partly support and protect the abdominal and thoracic viscera, and give rigidity and shape to the body in general. The amount of movement between any two vertebrae is small, but the combined movement permitted in all the intervertebral articulations is sufficient to allow considerable mobility of the whole body in any direction (Slijper, 1946). Bone consists of cells in a specialized intercellular organic matrix called osteoid, which is mineralized primarily by hydroxyapatite. The cells that direct the formation of cartilage and bone may be derived either from mesoderm or from neural crest (Hall, 1988; Noden & de Lahunta, 1985). The most abundant protein of the organic matrix of bone is type I collagen, which gives bone its structural support and strength. However, bone matrix contains numerous other matrix macromolecules, including collagen types III and V, proteoglycans, lipids, morphogenetic proteins, and enzymes as well as phosphoproteins and glycoproteins specific to bone, such as osteocalcin, osteonectin, and osteopontin. The function of these bone-specific proteins is a major area of current research, and in the future many new noncollagenous proteins of bone will undoubtedly be discovered. Cartilage, often a precursor of bone, has been reviewed in books by Hall (1977) and Hall and Newman (1991), who considered developmental and molecular aspects of cartilage (see Chapter 2 for a discussion of fetal bone development and illustrations of ossification sequences in various bones). Bone-forming cells, or osteoblasts, are capable of synthesizing extracellular collagenous and noncollagenous proteins and proteoglycans, the building blocks of bone matrix. They also respond to circulating hormones and produce growth factors that mobilize osteoclast precursor cells. Osteoblasts on the bone surface become osteocytes as they are surrounded by mineralized matrix (Bonewald, 2008). Each bone cell or osteocyte rests in a lacuna and has long branching processes that extend through canaliculi in the mineralized matrix to lacunae of neighboring cells. All bone-lining cells are interconnected and appear capable of maintaining active transport in calcium homeostasis. The formation of bone by osteoblasts and the resorption of bone by osteoclasts are linked, or “coupled,” in ways that are not completely understood. However, the controlling cell for bone remodeling, which goes on throughout life, is the osteoblast. Mechanical stress via muscle attachment, nutrition, vitamin D, calcitonin, parathormone, and sex hormones plays a great role in bone remodeling throughout life. In old age some bone cells die, whereas some become “uncoupled” and are not replaced, thus disrupting bone metabolism. This results in the thinning of cortical as well as trabecular bone. Resorption or deposition may be excessive and result in clinical problems, some of which still defy treatment. For a consideration of over-nutrition and skeletal disease, see Wu (1973). For an excellent review of bone cell biology, which the authors contend is still in its infancy, see Marks and Popoff (1988). Recent books on the osteoblast and osteocyte and the osteoclast are part of a seven-volume series titled Bone: A Treatise, edited by B. K. Hall (1989-1992). This is a timely update for the still useful four-volume, second edition of The Biochemistry and Physiology of Bone, by Bourne (1972-1976). In the new series by Hall, there will be 74 chapters by 127 authors. The fetal skeleton (see Chapter 2) is characterized by bones formed in membrane (intramembranous) that precede or accompany bones formed in cartilage (endochondral). Both intramembranous bone and endochondral bone are remodeled during development and form lamellar bone with haversian systems indistinguishable from each other. The terms membrane bone and cartilage bone refer to the primary tissue being mineralized. Almost all so-called cartilage bones begin their ossification beneath a perichondral membrane, followed by vascular invasion and endochondral ossification. Several membrane bones develop secondary cartilage after membranous ossification has begun. This secondary cartilage ossifies to form compact bone indistinguishable from the remainder of the structure. For further information, see Calcified Tissue Research, an international journal founded in 1967 and devoted to the structure and function of bone and other mineralized systems, and Developmental and Cellular Skeletal Biology by Hall (1978). The bones of the face and dorsum of the cranium develop in sheets of connective tissue, not in cartilage. This type of bone formation is known as intramembranous ossification. Osteoblasts and osteoclasts continue to be the laborers in this activity. The compact bone formed by the periosteum is identical with membrane bone in its elaboration. Bony tissue of either type is capable of growing in any direction. The jaws and hyoid arches are preceded by cartilages, which are derived from the neural crest. In the proceedings of the Third International Conference on Bone (Dixon et al., 1991) there are more than 50 papers that consider present methods for studying the growth of cartilage and bone. A common technique for studying developing cartilages and bones in the fetus is the use of color stains and the subsequent clearing of tissues. For cartilage, Alcian blue or toluidine blue is used to stain the mucopolysaccharide and for bone, alizarine red, combined with calcium to stain them red. Subsequent maceration of tissues with sodium or potassium hydroxide followed by clearing in glycerine, benzyl benzoate or ethylene glycol make cartilage and bone formation visible. Examples of such staining can be seen in Chapter 2. (Evans 1948, Orsini 1962, Crary 1962, Dingerkus & Uhler 1977, Kelly & Bryden 1983, Taylor & Van Dyke 1985). Bone is about one-third organic and two-thirds inorganic material. The inorganic matrix of bone has a microcrystalline structure composed principally of calcium phosphate. The exact constitution of the crystal lattice is still under study, but it is generally agreed that bone mineral is largely a hydroxyapatite with adsorbed carbonate. Some consider that it may exist as tricalcium phosphate hydrate with adsorbed calcium carbonate (Dixon & Perkins, 1956). The organic framework of bone can be preserved while the inorganic part is dissolved. A 20% aqueous solution of hydrochloric acid will decalcify any of the long bones of a dog in approximately 1 day. Such bones retain their shape but are pliable. A slender bone, such as the fibula, can be tied into a knot after decalcification. The organic material is essentially connective tissue, which on boiling yields gelatin. The nerves in bone are principally sensory and evidence has been accumulating that the nervous system plays a crucial role in remodeling of bone and the maintenance of bone mass (Martin & Sims, 2009). It is thought that approximately 10% of the human skeleton is replaced each year by remodeling. Both the central and sympathetic part of the peripheral nervous systems are believed to be involved in such regulation. Neuroendocrine controls include leptin, which inhibits bone formation. Sensory nerves carry impulses that may result in pain. Kuntz and Richins (1945) state that both the afferent and the sympathetic efferent fibers probably play a role in reflex vasomotor responses in the bone marrow. The skeleton of the vertebrate body serves four functions. 1. Bone forms the supporting and, in many instances, the protecting framework of the body. 2. Many bones serve as first-, second-, or third-class levers, owing to the action of different muscles at different times and to changes in the positions of force and fulcrum. Nearly all muscles act at a mechanical disadvantage. The speed at which the weight travels is in direct proportion to the shortness of the force arm, and this is determined by the distance of the insertion of the muscle from the joint, or fulcrum. 3. Bone serves as a storehouse for calcium and phosphorus and for many other elements in small amounts. The greatest drain occurs during pregnancy; conversely, the greatest deposition takes place during growth. In the large breeds, such as the Great Dane and St. Bernard, the skeleton is the system most likely to show the effects of a nutritional deficiency. Undermineralization of the skeleton is a common manifestation of underfeeding, improper feeding, or inability of the individual to assimilate food adequately. Overnutrition can result in a variety of skeletal diseases (Hedhammer et al., 1974). 4. Bone serves as a factory for red blood cells and for several kinds of white blood cells. In the normal adult it also stores fat. The axial skeleton is composed of the skull, hyoid bones, vertebral column, ribs, and sternum. The bones of the head compose the skull. It is divided into the bones of the cranium that surround the brain and the bones of the face that surround the eyes, and respiratory and digestive passageways (Figs. 4-3 to 4-5). The cranial cavity (cavum cranii), is separated from the cavity of the nose (cavum nasi) by a perforated plate of bone, the cribriform plate (see Fig. 4-9). Caudally the large opening through the occipital region, the foramen magnum, allows for the medulla oblongata to continue into the spinal cord along with its associated vessels. The bones of the ventral part (see Fig. 4-5) of the cranium, or basicranial axis, are pre-formed in cartilage, whereas those of the dorsum, or calvaria, are formed in membrane. A classic treatment of the development of the vertebrate skull by de Beer (1937) considers the homologies of skull components, compares chondrocrania, and discusses modes of ossification. The Mammalian Skull by Moore (1981) includes detailed descriptions of skull components, evolutionary changes, functional adaptations, and developmental anatomy. The bibliography is extensive. Hamon (1977) published a very detailed radiographic atlas of the dog skull. Skulls differ more in size and shape among domestic dogs than in any other mammalian species. For this reason, craniometry in dogs takes on added significance when characterizing specific breeds and crosses. Certain points and landmarks on the skull are recognized in making linear measurements and have been used by Stockard (1941) and others. The more important of these are (Figs. 4-6 to 4-8): Inion: Central surface point on the external occipital protuberance Bregma: Junction on the median plane of the right and left frontoparietal sutures, or the point of crossing of the coronal and sagittal sutures Nasion: Junction on the median plane of the right and left nasofrontal sutures Prosthion: Rostral end of the interincisive suture, located between the roots of the superior central incisor teeth Pogonion: Most rostral part of the mandible, at the intermandibular articulation, located between the roots of the inferior central incisor teeth Basion: Middle of the ventral margin of the foramen magnum The center of the external acoustic meatus: Although unnamed, this spot also serves as a reference point. Three terms are frequently used to designate head shapes (see Fig. 4-49): Dolichocephalic means “long, narrow-headed.” Breed examples are Collie and Russian Wolfhound. Mesaticephalic means a head of medium proportions. Breed examples are German Shepherd Dog, Beagle, and Setter. Brachycephalic means “short, wide-headed.” Breed examples are Boston Terrier and Pekingese. The face of the dog varies more in shape and size than does any other part of the skeleton. In brachycephalic breeds the facial skeleton is shortened and broadened. In some brachycephalic breeds, the English Bulldog, for example, the inferior jaw protrudes rostral to the superior jaw, producing the undershot condition known as prognathism of the mandible. Most other breed types have brachygnathic mandibles, that is, receding inferior jaws. Although brachygnathism of the mandibles is relative, both the Collie and the Dachshund frequently exemplify this condition to a marked extent. Stockard (1941) demonstrated that discrepancies in the pattern between the superior and the inferior jaws in the dog are inherited and develop as separate and independent characters. This can lead to marked disharmonies in facial features and dental occlusion, as was shown by the many crosses he made between purebred dogs. In the cross between the Basset Hound and the Saluki, two dogs with different skull proportions but without abnormally dissimilar jaws, some of the F2 hybrids showed the independent inheritance of superior and inferior jaw features. When one pup can inherit the muzzle and superior jaw of one parent and the inferior jaw from the other, it can have serious effects on dental occlusion and thus mastication, tooth loss, prehension, and so on. Occasionally, breed-specific features are accentuated in the crossbred dog so that minor aberrations become major features. Photographs of a variety of crosses of purebred dogs can be found in Stockard’s memoir (1941). Included are such crosses as Basset Hound–Shepherd, Basset Hound–Saluki, Basset Hound–English Bulldog, Dachshund–Boston Terrier, Dachshund–French Bulldog, Dachshund–Brussels Griffon, Pekingese–Saluki, Dachshund–Basset Hound, and Dachshund–Pekingese. Table 4-2 shows average measurements in millimeters taken from randomly selected adult skulls of the three basic types. From these data it can be seen that the greatest variation in skull shape occurs in the facial part. In making comparisons of skull measurements it is essential that the overall size of the individuals measured is taken into consideration. As a rule the dolichocephalic breeds are larger than the brachycephalic, whereas the working breeds fall in the mesaticephalic group, and these as a division have the greatest body size. The only measurement in which the brachycephalic type exceeds the others, in the small sampling shown, is facial width. To obviate the size factor among the breed types, indices are computed (Table 4-3). These indicate relative size and are expressed by a single term representing a two-dimensional relationship. The cranial index is computed by multiplying the cranial width by 100 and dividing the product by the cranial length. Skull and facial indices are computed in the same manner. Stockard (1941) found rather consistent differences between the sexes in most breeds, suggesting an endocrine influence for the differential structural expression. TABLE 4-2 Average Measurements of Three Skull Types TABLE 4-3 From Stockard CR: The genetic and endocrinic basis for differences in form and behavior, American Anatomy Memoir 19, Philadelphia, 1941, Wistar Institute of Anatomy and Biology. Trouth et al. (1977) have devised a morphometric index for determining the sex of a dog from the skull. On the ventral surface, in the basioccipital region there is a triangular area that extends from the basion to a line joining the medial points of the two tympanooccipital fissures. In the male it appears narrow and elevated. In the female skull the rostral half of the basioccipital area is wider and flat. Their formula for the sex index is Differences among the breeds in facial skeletal development are the most salient features revealed by craniometry. The face is not only short in the brachycephalic breeds but also is actually wider than in the heavier, longer-headed breeds. These data do not show that appreciable asymmetry exists, especially in the round-headed types. Even though the cranium varies least in size, it frequently develops asymmetrically. The caudal part of the skull is particularly prone to showing uneven development. The further a breed digresses from the ancestral wolf type (Suminski 1975, compares the wolf and dog skulls), the more likely are distortions to be found. This is particularly true of the round-headed breeds. The appearance of the English Bulldog is produced by the prognathic condition of the inferior jaw as well as the brachygnathic condition of the superior jaw. This structural disharmony results in poor occlusion of the teeth. Stockard (1941) found that the formation of the Bulldog type of skull results from a defective growth reaction of the basicranial physeal cartilages. This defective growth is foreshadowed by a deficiency in the cartilaginous matrix that is the precursor of the basioccipital and basisphenoid bones themselves. An early ankylosis of these growth cartilages (chondrodystrophy) causes the shortening of the basicranial axis. On sagittal section (Fig. 4-9) the limits of the cranial and facial portions of the skull are clearly demarcated by the cribriform plate of the ethmoid. Cranial capacity may vary between breeds and can be measured by filling the crania with mustard seed after the foramina have been closed with modeling clay, and then determining the volume of seed used. Average Boston Terrier skulls held 82 cc. A sampling of skulls of medium size and medium length showed an average capacity of 92 cc; the average skull capacity of the crania of the Russian Wolfhound and of the Collie was 104 cc. Wayne (1984) studied the morphologic similarity of skulls in wild and domestic canids. The names of the individual bones making up the 50 that compose the skull are listed in Box 4-1. Lateral and ventral views of an “exploded skull” showing the individual bones in relation to one another appear as Figures 4-42 and 4-45. The occipital bone (Figs. 4-5, 4-10, 4-11, 4-44 and 4-48) forms a ring, the foramen magnum, around the junction of the medulla oblongata and the spinal cord. The ring develops from four centers: a squamous part dorsally, two lateral condylar parts, and a basilar part ventrally. A keyhole-shaped notch may be present dorsally (Fig. 4-12). This normal feature is common in the brachycephalic toy breeds (Watson, 1981). The squamous part (squama occipitalis), also known as the supraoccipital, is the largest division. This bone forms the dorsal border of the foramen magnum and hides the cerebellum. An unpaired, median, interparietal bone (Fig. 2-39) makes its appearance on about the forty-fifth day of gestation. It is superficial to the paired parietal bones and to the supraoccipital bone. Usually, it fuses with the dorsorostral border of the supraoccipital bone forming part of the saggital crest, but in some dogs, it remains as a separate entity, the os interparietale. Occasionally an unfused interparietal bone is found in an adult dog. It may be more apparent inside the cranium than externally. Erhart (1943) examined 127 dog skulls for the presence of a separate interparietal bone, and he found 17 examples in 33 brachycephalic skulls; 9 in 30 mesaticephalic skulls; and none in 64 dolichocephalic skulls. Of the 14 brachycephalic fetuses studied, 12 had a distinct ossicle, whereas only 1 of 5 dolichocephalic fetuses had an independent ossicle. In the Beagle fetuses studied by Evans (1974) there was always a separate median interparietal bone for a brief period that fused indistinguishably with the squamous part of the supraoccipital bone before birth. From the interparietal process arises the middorsal external sagittal crest (crista sagittalis externa), which, in some specimens, is confined to this bone. The rostral end of the interparietal process is narrower and thinner than the caudal part, which turns ventrally to form a part of the caudal surface of the skull. The nuchal crest (crista nuchae) marks the division between the dorsal and the caudal surfaces of the skull. It is an unpaired, sharp-edged crest of bone that reaches its most dorsal point at the external occipital protuberance. On each side it arches ventrally before ending on a small eminence located dorsocaudal to the external acoustic meatus. The external occipital protuberance (protuberantia occipitalis externa) is the median, triangular projection forming the most dorsocaudal portion of the skull. The external occipital crest (crista occipitalis externa) is a smooth median ridge extending from the external occipital protuberance to the foramen magnum. It is poorly developed in some specimens. Variations in the occipital bone are numerous. The foramen magnum varies in shape and is not always bilaterally symmetric (Figs. 4-12 and 4-13) (Simoens et al., 1994; Watson et al., 1989). The condyloid canal may be absent on one or both sides. Even when both canals are present, connections between the hypoglossal and the condyloid canals may fail to develop. The paracondylar processes may extend several millimeters ventral to the tympanic bullae so that they will support a skull without the mandibles when it is placed on a horizontal surface; conversely, they may be short, retaining the embryonic condition. The vermiform impression may be deep, causing a caudomedian rounded, thin protuberance on the caudal surface of the skull. The foramen for the dorsal sagittal sinus may be double. It is rarely median in position. A sutural bone may be present at the rostral end of the interparietal process.
The Skeleton
General
Classification of Skeletal Elements
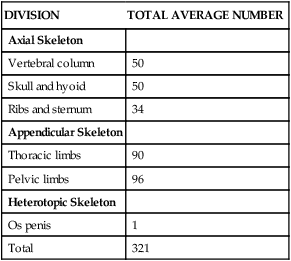
DIVISION
TOTAL AVERAGE NUMBER
Axial Skeleton
Vertebral column
50
Skull and hyoid
50
Ribs and sternum
34
Appendicular Skeleton
Thoracic limbs
90
Pelvic limbs
96
Heterotopic Skeleton
Os penis
1
Total
321
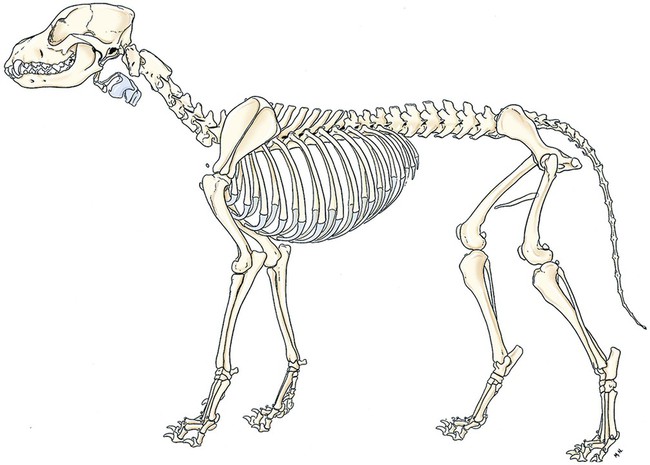
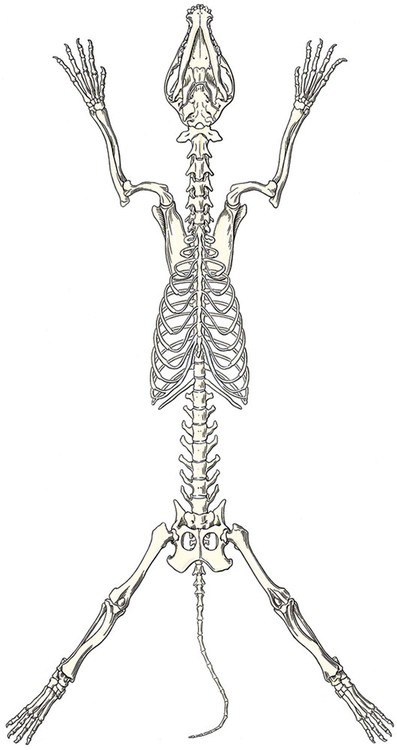
Classification of Bones According to Shape
Development of Bone
Physical Properties of Bone
Vessels and Nerves of Bone
Function of Bone
Axial Skeleton
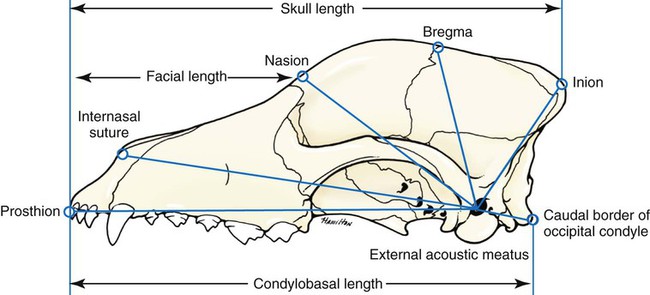
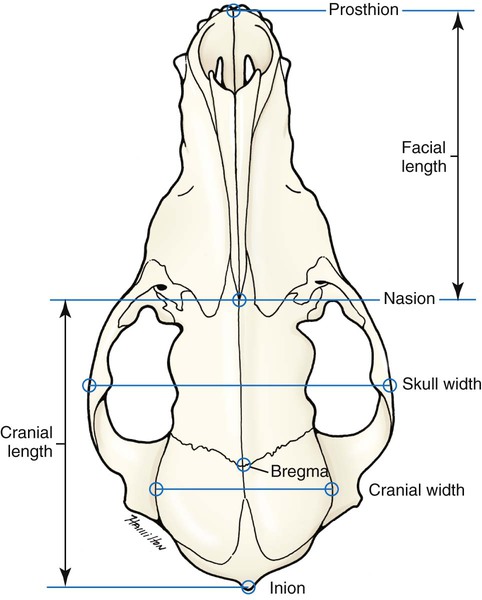
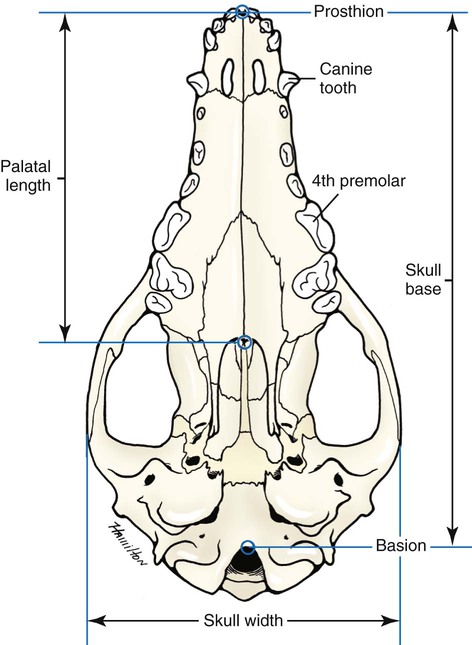
Skull
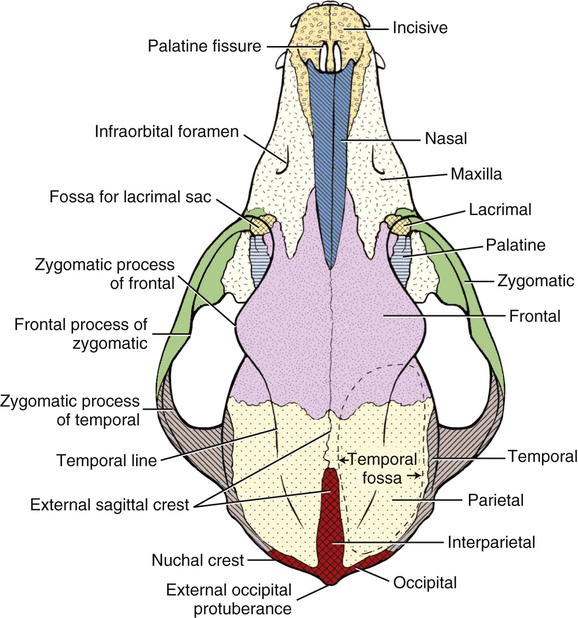
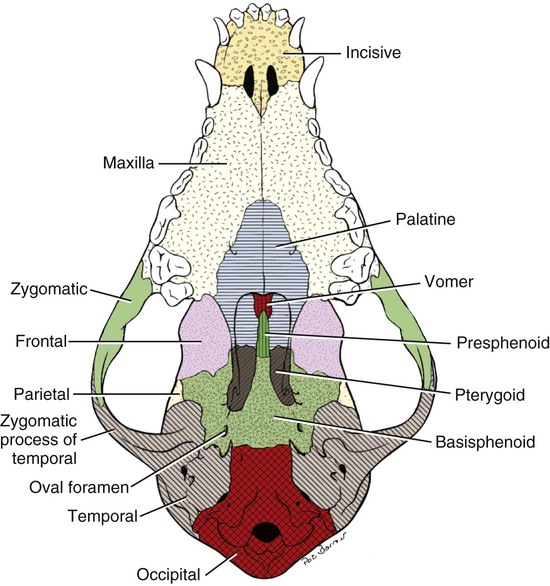
MEASUREMENT
BRACHYCEPHALIC
MESATICEPHALIC
DOLICHOCEPHALIC
Facial length
Nasion to prosthion
48 mm
89 mm
114 mm
Facial width
Widest interzygomatic distance
103 mm
99 mm
92 mm
Cranial length
Inion to nasion
99 mm
100 mm
124 mm
Cranial width
Widest interparietal distance
56 mm
56 mm
59 mm
Cranial height
Middle of external acoustic meatus to bregma
54 mm
60 mm
61 mm
Mandibular length
Caudal border of condyle to pogonion
85 mm
134 mm
163 mm
Skull length
Inion to prosthion
127 mm
189 mm
238 mm
Skull width
Widest interzygomatic distance
103 mm
99 mm
92 mm
Indices
Skull base length
Basion to prosthion
107 mm
170 mm
216 mm
Skull index
81
52
39
Cranial index
57
56
48
Facial index
215
111
81
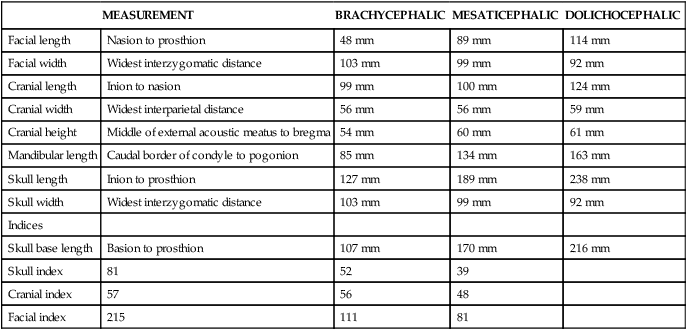
GERMAN SHEPHERD DOG
SALUKI
ENGLISH BULLDOG
PEKINGESE
BRUSSELS GRIFFON
Cranial index
51
64
69
84
84
Skull index
56
56
107
107
103
Palatal index
60
57
122
122
125
Snout index
60
53
171
179
183
Groups of breeds having similar types of skulls:
I. German shepherd dog
II. St. Bernard
III. English Bulldog
Foxhound
Great Dane
French Bulldog
Saluki
Dachshund
Boston Terrier
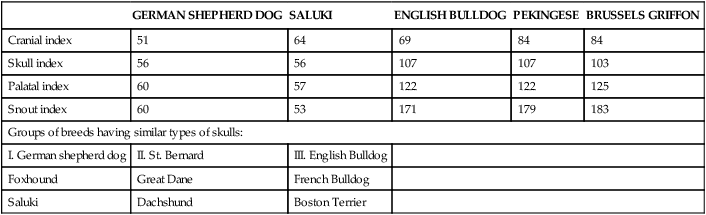
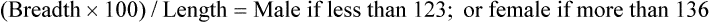
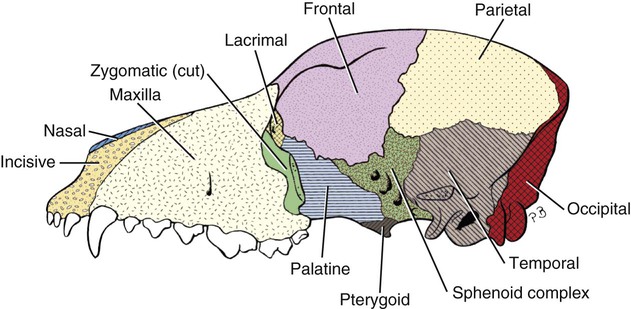
Bones of the Cranium
Occipital Bone
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


The Skeleton
Only gold members can continue reading. Log In or Register to continue