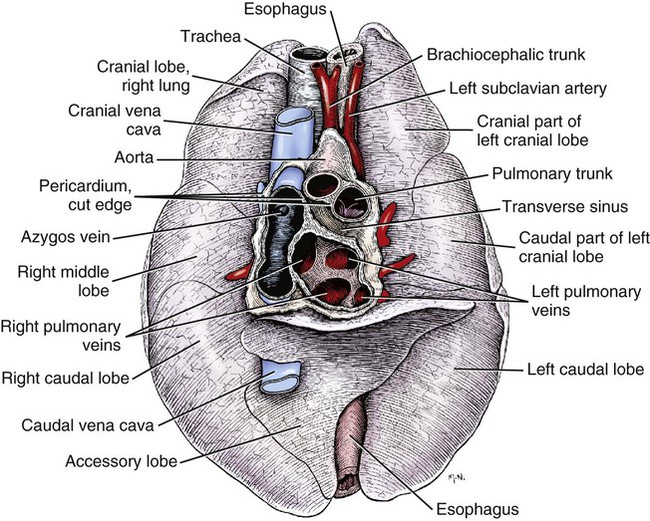
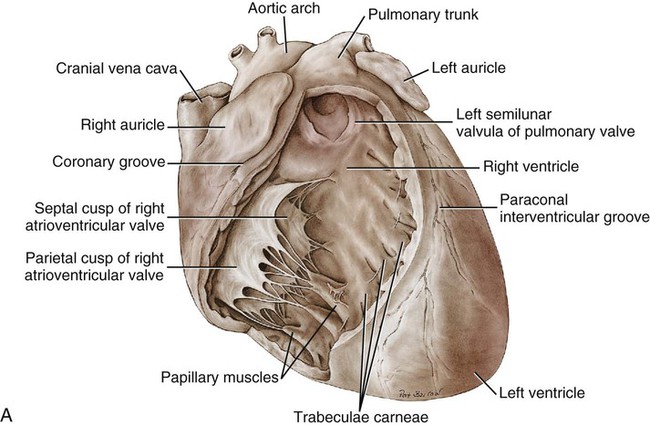
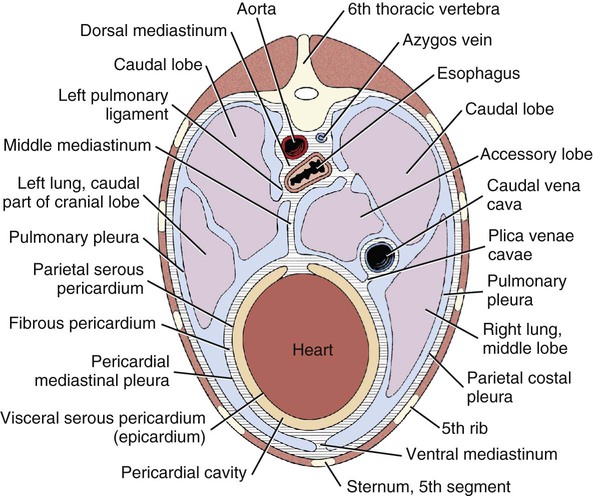
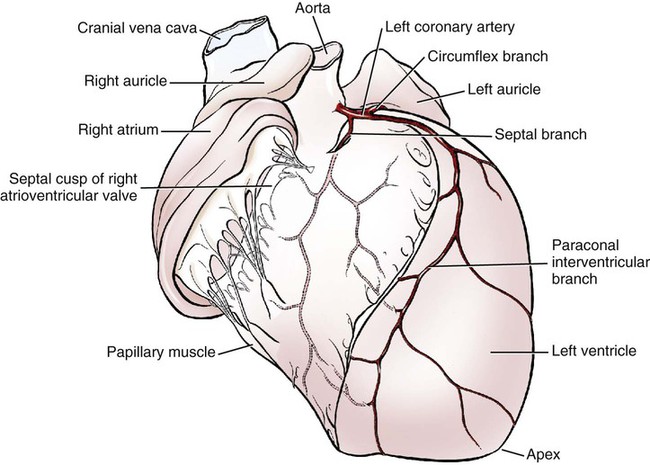
The fibrous pericardium (pericardium fibrosum) (Fig. 11-1) is a thin, tough sac that contains the serous pericardium, a small amount of fluid and the heart. The greater part of its outer surface is covered by and adhered to the pericardial mediastinal pleura. In young dogs the thymus is in contact with a variable portion of its cranial surface. The inner surface of the fibrous pericardium is intimately lined by the parietal layer of the serous pericardium. The base of the fibrous pericardium is continued on the adventitia of the great arteries and veins that leave and enter the heart. It blends with the adventitia of these vessels. Its apex is continued to the ventral part of the muscular periphery of the diaphragm in the form of a dorsoventrally flattened band of yellow elastic fascicles, the phrenicopericardiac ligament (lig. phrenicopericardiacum). This is nearly 1 cm wide, less than 1 mm thick, and approximately 5 mm long. The serous pericardium (pericardium serosum) (Fig. 11-1) forms a closed cavity into which approximately one half of its wall is invaginated by the heart to form its visceral layer, the smooth, outer covering of the heart also known as the epicardium. The uninvaginated part forms the parietal layer as it covers the inner surface of the fibrous pericardium. The pericardial cavity (cavum pericardii) (see Fig. 11-1) is located between the two layers of the serous pericardium. It is the smallest of the serous body cavities and, unlike the others, contains 0.3 to 1 mL of a clear, light yellow fluid, the liquor pericardii. The visceral layer (lamina visceralis) of the serous pericardium, or epicardium, is attached firmly to the heart muscle, except primarily along the grooves where fat and the coronary vessels or their branches intervene. Its smooth mesothelial surface is underlaid by a stroma that contains elastic fibers. The division between the parietal and visceral layers of the serous pericardium is marked by an undulating line that follows the highest part of the pericardial cavity around and across the base of the heart. The aorta and pulmonary trunk, the two great arteries leaving the heart, are united by a tube of epicardium and areolar connective tissue at their origins so that, caudal to them, a part of the pericardial cavity curves transversely across the base of the heart. This is the transverse sinus of the pericardium (sinus transversus pericardii) (Fig. 11-2), a U-shaped passage between the right and left sides of the pericardial cavity. This sinus passes between the aorta and pulmonary trunk cranially and the pulmonary veins caudally. The heart (cor) (Figs. 11-3, 11-4, and 11-5) is the muscular pump of the cardiovascular system. The musculature and conducting system of the heart are spoken of collectively as the myocardium (Langer & Brady, 1974). For references on the heart, blood vessels, and circulation, see Chevalier (1976), Cliff (1976), and Abramson (1976). The heart is cone-shaped and obliquely placed in the thorax so that its base (basis cordis) faces dorsocranially and its apex (apex cordis) is directed ventrocaudally and to the left side. A small, roughly triangular area of its dorsocaudal surface adjacent to the apex is related to the diaphragm. The large remaining part of its circumference is largely covered by the lungs and faces the sternum and ribs. The left and right ventricles are separated from each other internally by a transverse curved oblique interventricular septum (septum interventriculare) that extends from a cranioventral position on the left to a caudodorsal position on the right. On the surface of the heart these positions of the septum are marked by the paraconal interventricular groove (sulcus interventricularis paraconalis) on the left and the subsinuosal interventricular groove (sulcus interventricularis subsinuosus) on the right. The atria are separated from each other by a thin muscular interatrial septum (septum interatriale) that is also transversely curved similar to the interventricular septum but has no surface markings. The atria are the blood receiving chambers and the ventricles are the blood pumping chambers. The left dorsal, lateral and caudal (caudodorsal) parts of the heart consist of the left atrium (atrium sinistrum) and the left ventricle (ventriculus sinister). The left atrium receives blood from the lungs and the left ventricle pumps it to all parts of the body (systemic circulation). The right dorsal, lateral and cranial (cranioventral) parts of the heart consist of the right atrium (atrium dextrum) and right ventricle (ventriculus dexter). The right atrium receives blood from all parts of the body and the right ventricle pumps it to the lungs (pulmonary circulation). The dog heart is frequently used in studies of cardiac hypertrophy, and several surveys have been made of the normal values. Herrmann (1925) includes data on 200 dogs and cites heart weight to body weight ratios averaging 8.10 g per kg of body weight for males and 7.92 for females. Northrup et al. (1957) analyzed Herrmann’s data and found that the sex differences were not significant. However, when they studied the heart weight to body weight ratios of an additional 346 adult dogs and 135 pups, they found that females have significantly smaller ratios than males. Small adults were found to have higher ratios than large adults, although pups had significantly smaller ratios than those in any adult category. The average ratio for 169 adult males was 7.74, and for 177 females it was 7.56. The range for 346 adult dogs was 4.53 to 11.13 g per kg. Schoning et al. (1995) found the heart weight to body weight ratio to be 1.3% for males and 1.2% for females in the 230 racing and nonracing Greyhounds that they studied. House and Ederstrom (1968) found that heart weight to body weight ratios increased during postnatal development from 7.17 g per kg in the newborn to 8.87 g per kg in adults. Latimer (1961) has presented data on the ratios between the weights of the walls of the right and left ventricles in 46 dogs. The right ventricular wall accounted for 22% of the total heart weight, the left ventricular wall for 39%. In the average young dog the heart weight is approximately 1% of the body weight. Schneider et al. (1964) compared the hearts of 40 racing Greyhounds with 60 mongrel dogs as to heart weight/body weight ratios and components of the electrocardiogram. The heart weight/body weight ratio of the Greyhounds was 1.25 g, with a heart rate of 115 beats per min. The ratio for mongrel dogs was 0.80, with a heart rate of 86 beats per min. The Greyhound has been bred and trained for speed and endurance, and it would be expected that the heart would be proportionally larger than that of a mongrel. Radiologists use a vertebral scale system to measure relative heart size on radiographs. Sleeper and Buchanan (2001) used this system to determine relative heart size in 11 clinically normal puppies to assess whether relative heart size changes with growth. They concluded that vertebral heart size measurements in puppies are within the reference range for adult dogs and do not change significantly with growth to 3 years of age. The interventricular grooves are indistinct surface markings of the separation of the right and left ventricles. Because they neither indent the substance of the heart nor contain as much fat as does the coronary groove, vessels are visible in them; however, most vessels stream toward the apex on the surface of the ventricles, rather than following the interventricular grooves. Obliquely traversing the left cranioventral surface of the heart is the paraconal interventricular groove (sulcus interventricularis paraconalis) (see Fig. 11-4), formerly known as the left, ventral, or cranial longitudinal sulcus or groove. It begins on the caudodorsal side of the conus arteriosus, where it is covered by the auricular portion of the left atrium; it ends before reaching the apex of the heart. The subsinuosal interventricular groove (sulcus interventricularis subsinuosus) (see Fig. 11-5), formerly known as the right, dorsal, or caudal longitudinal sulcus, is a short, straight, shallow furrow that marks, on the dorsocaudal surface of the heart, the approximate position of the interventricular septum. The right atrium (atrium dextrum) (Fig. 11-6) receives the blood from the systemic veins and most of the blood from the heart itself. It lies dorsocranial to the right ventricle. It is divided into a main part, the sinus venarum cavarum, and a blind part, which projects cranially, ventrally, and left, the right auricle (auricula dextra). The main openings of the right atrium are four in number. The coronary sinus (sinus coronarius) is the smallest of these and enters the atrium caudally from the left. Dorsal to it is the large caudal vena cava (vena cava caudalis), which enters the heart from caudal. The caudal vena cava returns blood from the abdominal viscera, part of the abdominal wall, and the pelvic limbs. The cranial vena cava (vena cava cranialis) is approximately the same size as the caudal vena cava. It enters the heart from dorsal and cranial. In the dog the azygos vein usually enters the cranial vena cava dorsally at the base of the heart, although occasionally it enters the atrium directly. The azygos vein drains blood back to the heart from part of the lumbar region and the caudal three fourths of the thoracic wall. The cranial vena cava returns blood to the heart from the head, neck, thoracic limbs, the ventral thoracic wall, and the adjacent part of the abdominal wall. The right atrioventricular orifice (ostium atrioventriculare dextrum) is the large opening from the right atrium into the right ventricle. This opening and the valve that guards it are described with the right ventricle. The interatrial septum (septum interatriale) extends from the level of the coronary sinus to the opening into the right auricle. On its right side is a transverse ridge of tissue placed between the two caval openings. This is the intervenous tubercle (tuberculum intervenosum). It diverts the converging inflowing blood from the two caval veins into the right ventricle. Just caudal to the intervenous tubercle on the medial wall of the atrium is a slitlike depression, the fossa ovalis, which varies from one to several millimeters in depth. Dias et al. (1979) summarize the appearance of the fossa ovalis in 77 adult dog hearts. All were translucent when illuminated, and the shape varied from piriform, which was most common, through triangular, elliptical, oval, kidney-shaped, and round. The crescent-shaped ridge of muscle that projects from the caudal side of the intervenous tubercle and deepens the fossa is the limbus fossae ovalis. In the fetus there is an opening at the site of the fossa, the foramen ovale, which allows blood to pass through the interatrial septum from the right to the left atrium. The foramen usually closes during the first few postnatal weeks. Even though a small anatomic opening frequently persists, it is closed physiologically because the obliquity of the foramen and the thin valve of the foramen ovale enable the greater pressure in the left atrium to close the passage. The sinus venarum cavarum is the smooth walled portion of the right atrium between the caval openings and the right atrioventricular opening. It is bounded by the interatrial septum and the crista terminalis. The fibrous base of the heart, or “cardiac skeleton” (Fig. 11-7B), is the fibrous tissue, containing some cartilage, which separates the thin atrial musculature from the much thicker muscle of the ventricles. Only the special neuromuscular tissue, the atrioventricular bundle, extends continuously through the fibrous base from the atria to the ventricles. Baird and Robb (1950) have reconstructed the principal parts of the conduction system of a puppy’s heart. Their dissections and reconstruction show the close spatial relationship that exists between the primary conduction and supporting tissues of the dog’s heart. The cardiac skeleton is in the form of four narrow fibrous rings (anuli fibrosi), one surrounding each atrioventricular orifice, and two scalloped cuffs, or rings, one surrounding each arterial orifice. These provide attachment for the respective valves. Of the two arterial rings, the aortic fibrous ring (anulus fibrosus aorticus) is the better developed. Peripherally the collagenous fibers that form it give way to the yellow elastic fibers composing the tunica media of the wall of the ascending aorta. There are three points on its aortic margin, each of which conforms to the attachments of two adjacent semilunar valvulae of the aortic valve. The projections between the attachments of the septal and right and the septal and left semilunar valvulae are best developed and are composed of hyaline cartilage. Between the points, the margin of the aortic fibrous ring is semilunar so that its scalloped circumference is in the form of three arcs. Between the aortic fibrous ring and the fibrous rings of the left and right atrioventricular rings, the fibrous base of the heart forms two triangular-shaped bundles of fibrous tissue. The left fibrous trigone (trigonum fibrosum sinistrum) is smaller than the right and lies in the triangle between the aortic and left atrioventricular ostia to the left of the right fibrous trigone. The right fibrous trigone (trigonum fibrosum dextrum) lies between the two atrioventricular ostia. Both trigones contain cartilage and are united through the medium of the opposed aortic and left atrioventricular fibrous rings. In the ox and horse the trigone ossifies in part. Sandusky et al. (1979) found that chondrocytes were common in the central fibrous body and root of the aorta and that bone had formed in the bundle of His in 8 of 40 dogs they examined. The atrioventricular fibrous rings (anuli fibrosi atrioventriculares) (Fig. 11-7B) are thin rings of collagenous tissue to which the muscle fibers of the atrial and ventricular walls attach. From their endocardial edges emanate the atrioventricular valves. Although the proximal parts of the ventricular musculature attach to their distal ventral surfaces, the bulk of the fibers of this muscle at its origin lie peripheral to the rings as the muscle tissue bulges proximally after arising from them. These fibrous rings are so delicate they are best seen in longitudinal sections of the heart cut through the atrioventricular junctions. The ventricles form the bulk of the heart. Together they form a conical mass, the apex of which is also the apex of the left ventricle. The right ventricle is dextral, ventral, and cranial to the left and arciform in shape (Fig. 11-8). The right ventricle (ventriculus dexter) (Fig. 11-9) receives the systemic blood from the right atrium and pumps it to the lungs. Its outline is in the form of a curved triangle, as it is molded on the surface of the caudodorsally lying, conical-shaped left ventricle. It is crescentic in cross section, and its long axis extends from the subsinuosal interventricular groove to the pulmonary trunk. The interior of both ventricles contains muscular ridges and projections with small, usually deep and oblong depressions between. The conical-shaped muscular projections in each ventricle that give rise to the chordae tendineae are called papillary muscles (musculi papillares). They are the most conspicuous features of the ventricular walls. There are usually three main papillary muscles in the right ventricle, although great variation exists (see Fig. 11-9A). Typically they arise from the apical third of the septal wall, 1 to 3 cm from its junction with the outer wall. Their branched chordae tendineae, which prevent eversion of the valve, go to the free border and adjacent ventricular surface of the parietal cusp of the right atrioventricular valve. Commonly the papillary muscle that lies farthest cranial is larger than the others, and, when it is, its apex is usually bifid. The papillary muscle that lies farthest caudal may be bifid also. In such specimens the middle muscle is absent. A single compound papillary muscle may replace the three main ones. Other variations are common. Near the angle formed by the septal wall and the outer wall, and caudodorsal to the most caudal large papillary muscle, is a small, blunt to conical papillary muscle that gives rise to chordae tendineae going to the most caudodorsal part of the parietal cusp. Truex and Warshaw (1942), in the 12 dogs they studied, found only three large papillary muscles from the septum and a small constant papillary muscle of the conus. The numerous chordae tendineae that go to the septal cusp arise from the septal wall directly or from muscular ridges or papillae located peripheral to the septal cusp, when this valvula lies against the septum. The trabecula septomarginalis dextra (see Fig. 11-8), formerly called moderator band, is a branched or single muscular strand that extends across the lumen of the right ventricle. It usually leaves the septal wall of the right ventricle near or from the base of the largest papillary muscle and runs to the outer wall. The extremity of the trabecula usually branches repeatedly as it blends with the muscular ridges of the outer right ventricular wall. It serves for the passage of cardiac conduction fibers from the right branch of the atrioventricular bundle across the lumen of the cavity. Instead of the usual single band, there may be two or more anastomosing strands forming a loose plexus. The left ventricle (ventriculus sinister) (Fig. 11-10) is conical in shape, with its apex forming the apex of the heart. It receives the oxygenated blood from the lungs by way of the left atrium and pumps it to the body through the aorta. The left atrioventricular ostium (ostium atrioventriculare sinistrum) is the large opening between the left atrium and the left ventricle closed by the left atrioventricular valve. The aortic ostium (ostium aortae), located near the center of the base of the heart, is the opening from the left ventricle into the ascending aorta. The left ventricle is characterized by its thick wall, conical shape and two large papillary muscles. Its wall is three or four times thicker than that of the right ventricle. Truex and Warshaw (1942), in 12 specimens, found the mean thickness of the left and the right ventricle to be 13 and 4.2 mm, respectively. The two large papillary muscles (musculi papillares) of the left ventricle come from its outer wall. They are thick, smooth rolls of myocardium, which have compound apices and give rise to the stout chordae tendineae of this chamber. The subauricular papillary muscle (musculus papillaris subauricularis) lies near the subsinuosal interventricular groove; the subatrial papillary muscle (musculus papillaris subatrialis) is closer to the paraconal interventricular groove. Adjacent to the subatrial papillary muscle, near its attachment, is a fine network of muscular strands that come from the septal wall. From findings in similar strands from other species (Truex & Warshaw, 1942), it is probable that these contain fascicles of cardiac conduction fibers derived from the left branch of the atrioventricular bundle en route to the subatrial papillary muscle and general ventricular musculature, and represent the trabeculum septomarginalis sinistrae. The strand nearest the atrioventricular opening is larger than the others and extends obliquely to the subatrial papillary muscle from the septal wall. It may be the only one present. Near the apex of the ventricle some of the fine threads that compose this network extend under the endocardium that covers the crests of the muscular ridges. Other threads bridge the sulci between the larger trabeculae carneae in their courses to the subatrial papillary muscle. The chordae tendineae from the subatrial papillary muscle go to both the septal and the parietal cusps of the left atrioventricular valve. Those that go to the septal cusp are shorter and arise closer to the ventricular wall than those that go to the parietal cusp of the valve. The subauricular papillary muscle is separated from the subatrial papillary muscle by a deep cleft. The origin, course, and termination of the chordae tendineae from this muscle are similar to those of the chordae tendineae from the subatrial muscle. Both papillary muscles extend to within a few millimeters of the apex of the ventricle. The musculature of the ventricles in the dog was described by Thomas (1957). There are superficial, middle, and deep layers. All muscle fasciculi of the superficial layer arise from the fibrous base and return to it. The superficial layer is common to both ventricles. When the heart is viewed from the base, these bundles run toward the apex, showing a clockwise twist. At the apex of the heart they turn in and run toward the base in such a manner that they cross, at right angles, the superficial fibers running ventrally. The superficial fibers penetrate the middle layer of the right ventricle to become its deep layer, after which they are disposed as on the left side. They form the papillary muscles. The middle layer forms the bulk of the ventricular walls. These are spiral or circular muscle masses that interdigitate between the two chambers. They primarily decrease the size of the lumen of the ventricles, and the superficial and deep layers shorten and twist the organ. The apex of the heart is quite thin, being formed by muscle fasciculi of the superficial layer of the left ventricle as they swirl in figure-of-eight fashion to form the apex of the chamber. The atrioventricular valves (valvae atrioventriculares) (Figs. 11-7, 11-9, and 11-10) are irregular, serrated cusps that are located in the atrioventricular ostia. They are the intake valves to the ventricles. They prevent blood from returning to the atria during the systolic phase of the heart beat. Peripherally, they attach to the fibrous rings that separate the musculature of the atria from that of the ventricles. When the ventricles contract, the valves are kept from being pushed into the atria by the chordae tendineae. The chordae tendineae attach to the ventricular surfaces of the valvulae. The largest cords can be followed as ridges under the endocardium to their attached borders, whereas the thinnest cords go to the points of the serrations and disappear. The medium-sized cords go as far as the middle part of the ventricular surfaces of the valvulae before blending with the stratum proprium. Although some blood vessels have been described in the valves adjacent to their attached borders, the bulk of the nutrition of the valves is derived from the free blood in the heart. Smith (1971) found a nerve network in the atrioventricular and semilunar valves of the dog that concentrated near the points of attachment of the chordae tendineae. Nerves composing the atrioventricular valve plexus were derived from the atrial subendocardial network. In general, the right atrioventricular valve was better innervated than the left atrioventricular valve. The aortic valve (valva aortae) (Figs. 11-7 and 11-10) consists of right, left, and septal semilunar cusps, or valvulae (valvulae semilunares dextra et sinistra et septalis). These consist of a fibrous tissue stroma covered on each surface by endothelium. Opposite their free borders they are attached to the aortic fibrous ring. In the middle of the free borders of the semilunar cusps are nodules (noduli valvularum semilunarium). Extending from each nodule toward the periphery of the valvulae are the lunulae (lunulae valvularum semilunarium). These represent the areas of contact with the adjacent cusps when the valve is closed. The nodules close the space that would otherwise be left open by the coming together of the three contiguous arcs. On the vessel side, or peripheral to each of the semilunar cusps, the wall of the aorta is dilated to form the three aortic sinuses (sinus aortae). These, like the cusps, are right, left, and septal in position, with the right and left coronary arteries leaving the right and left sinuses. The widening of the base of the ascending aorta, formed by the aortic sinuses, is the aortic bulb (bulbus aortae). At the end of systole, or ventricular contraction, the pressure in the aorta is greater than that in the left ventricle, and a back pressure is developed that closes the valve and dilates the sinus. The coronary arteries leaving the sinus are affected by the changes in pressure. Boucek et al. (1964) described and illustrated the functional anatomy of the region of the aortic sinus, coronary ostia, and ascending aorta. They correlated their radiographs with different phases of the cardiac cycle and consider the rotation of the aorta during systole. The valve of the pulmonary trunk (valva trunci pulmonalis) (Figs. 11-7 and 11-9) lies cranial and to the left of the aortic valve and is similar to it in construction. Because the blood pressure developed in the pulmonary trunk that it guards is not as great as that in the aorta, the development of the cusps and related structures is not as extensive. All parts present in the aortic valve are represented in the pulmonary valve. The valve of the pulmonary trunk consists of right, left and intermediate semilunar cusps, or valvulae (valvulae semilunares dextra et sinistra et intermedia). A three-dimensional description of the distribution and organization of the canine intrinsic cardiac nervous system was developed by Yuan et al. (1994). They consistently found distinct epicardial ganglionated plexuses in four atrial and three ventricular regions, with occasional neurons being located throughout atrial and ventricular tissues in the 67 mongrel dog hearts studied. The first atrial plexus extended from the ventral to the dorsal surface of the right atrium, the second was located in the fat on the ventral surface of the left atrium, the third plexus was located on the middorsal surface of the two atria and the fourth extended from the base of the cranial vena cava to the dorsal caudal surface of both atria. Ventricular plexuses surrounded the origin of the aorta and extended along the coronary arteries. Pauza et al. (1999) reported similar results for the 36 mongrel dog hearts they examined. Ursell et al. (1990) showed that the sympathetic innervation of the heart developed progressively after birth and reached maturity by 2 months of age. No part of the conducting system of a preserved dog’s heart can be adequately identified in gross dissection without special preparation. The conducting system consists of three parts, which are closely integrated physiologically: (1) the sinoatrial node, (2) the atrioventricular node, and (3) the atrioventricular bundle. Baerg and Bassett (1963) described a method to stain the conduction tissue with palladium iodide. The sinoatrial node (nodus sinuatrialis) appears to be the center that initiates the heartbeat and also regulates the interval between beats. It is located in the terminal crest at the confluence of the cranial vena cava, sinus venarum cavarum, and auricular orifice. When it is destroyed experimentally, the heartbeat slows or stops. The sinoatrial node is composed of cardiac conduction fibers (myofibra conducens cardiaca) formerly known as Purkinje fibers. These fibers are little modified from those that compose the atrioventricular conduction system. Nonidez (1943) has shown that both the sinoatrial and the atrioventricular nodes are supplied by postganglionic parasympathetic nerve terminals. The atrioventricular bundle (fasciculus atrioventricularis) begins as a mass of cardiac conduction fibers known as the atrioventricular node (nodus atrioventricularis). This is approximately 1.5 mm in diameter in the dog, according to Baird and Robb (1950), and shows little histologic differentiation from the bundle. Nonidez (1943) demonstrated that the atrioventricular node received a richer parasympathetic innervation than did the sinoatrial node. The atrioventricular node begins in the septal wall of the atrium approximately 5 mm cranioventral to the opening of the coronary sinus and craniodorsal to the septal cusp of the right atrioventricular valve. From this apparently blind beginning, the atrioventricular bundle runs cranial and ventral through the fibrous base of the heart. As it does so it divides into right and left branches. These lie closely under the endocardium of the septal wall of the right and left ventricles. The right septal branch crosses the cavity of the right ventricle in the septomarginal trabecula of this chamber. It arborizes in the parietal ventricular wall of the right ventricle, where it ends. The left septal branch is more diffuse than the right one and partly traverses the cavity of the left ventricle in the left septomarginal trabeculae to the parietal wall of this chamber in bundles, which are smaller and more branched than those on the right side. The ramification of the conduction system of the heart is more complicated than the previous description indicates. Baird and Robb (1950) found that there were transitions from the atrioventricular node to the atrial muscle and that various parts of the atrioventricular bundle blended with the general ventricular musculature. Abramson and Margolin (1936) wrote that in the dog, as in other species, the myocardial branches, as they leave the subendothelial plexus, tend to pass perpendicularly to the endocardium in the left ventricular wall and obliquely in the right ventricular wall. They further state that the interventricular septum is traversed by myocardial conduction fibers that arise from the adjacent subendothelial conduction fiber networks. Racker (1989) was able to demonstrate at least three discrete atrioventricular bundles of myocardium that join the atrial end of the atrioventricular node via a proximal atrioventricular bundle. Her study was to determine the architecture of the atrioventricular junctional region, and she was able to confirm internodal tracts of myocardial fibers. Halpern (1955) described the blood supply to the atrioventricular system of the dog heart. The interatrial septum is supplied by three arteries: two from the right coronary artery and one from the left. Anastomoses between these branches occur in the septum. The common bundle was supplied by the septal artery and dorsal left atrial artery; both are branches of the left coronary artery. The accessory ventral right atrial branch of the right coronary artery also participates. The right and left coronary arteries and their branches supply the muscle of the heart. They arise from the aortic bulb immediately distal to the aortic valve. Higginbotham (1966) investigated the question of variation in coronary artery patterns between closely related pedigreed dogs and mongrels. The conclusion was that there is as much, if not more, variation in purebred dogs. The right coronary artery (a. coronaria dextra) (Figs. 11-5 and 11-7A) is smaller than the left coronary artery and measures approximately 1.5 mm in diameter and 5 cm in length. It arises from the right sinus of the aorta and makes a sweeping curve to the right and ventrocranially, lying in the fat of the coronary groove. Its initial part is bounded by the pulmonary trunk and the conus arteriosus cranially and on the left, and dorsally it is covered by the right auricle. The right coronary artery supplies the bulk of the parietal wall of the right ventricle. As a result of ligation studies, Donald and Essex (1954) found that an average of 66% of the parietal right ventricular wall normally receives its blood through the right coronary artery. According to Kazzaz and Shanklin (1950), the right coronary artery rarely extends as far as the borders of the right ventricle and in no case goes beyond them. It also sends branches to the right atrium, and small twigs to the initial parts of the aorta and pulmonary trunk. In 20% of specimens, according to Moore (1930), an accessory right coronary artery (a. coronaria dextra accessoria) arises closely adjacent to the main right coronary artery from the right sinus of the aorta and runs approximately 2 cm distally on the conus arteriosus, where it largely becomes dissipated. According to Pianetto (1939), there are four to nine ventricular branches of the main right coronary artery. Most of these are small vessels that arborize on and in the right ventricular wall. Tanaka et al. (1999) quantified the branching patterns of intramural and epicardial vessels and found that a typical arterial segment divided into nearly two equivalent branches. They run at right angles to the long axis of the cavity, and none extend as far as the subsinuosal interventricular groove. One of these, the right marginal branch (ramus marginalis dextra), is larger, longer, and more branched than the others. It supplies the middle part of the right lateral ventricular wall. The atrial branches from the right coronary artery are variable in development. Kazzaz and Shanklin (1950), Meek et al. (1929), Moore (1930), and Pianetto (1939) all describe an atrial branch, larger than the others, which leaves the distal half of the artery, traverses the sulcus terminalis and, as it does so, supplies the sinoatrial node. This vessel anastomoses with one or more atrial branches that arise from the circumflex branch of the left coronary artery. Usually one or two small atrial rami leave the right coronary artery both proximal and distal to the branch destined for the sinoatrial node. Mitsuoka et al. (1987) present a description of the technique for cannulation of the atrioventricular nodal artery and its anatomical variations in 30 dogs. The distal branches supply the caudal part of the right atrium adjacent to the coronary sulcus. A terminal twig ends on the caudal vena cava. The first branch or two that leave the dorsal surface of the right coronary artery supply the right auricle. The normal anastomoses between the right coronary artery and adjacent vessels are small. In a corrosion cast study of hearts from normal dogs, Noestelthaller et al. (2005) found inter- and intraarterial anastomoses in 8 out of 31 of the hearts that they studied. The left coronary artery (a. coronaria sinistra) (Figs. 11-3 through 11-5, 11-7A, and 11-11) is a short trunk approximately 5 mm long and nearly as wide. It always terminates in the circumflex and paraconal interventricular branches and, in many specimens, in a septal branch also. Noestelthaller et al. (2007) characterize three types of divisions: Type I, in which the short trunk divides into circumflex, paraconal interventricular, and septal branches at the same point; Type II, in which the septal branch comes off the paraconal interventricular branch; and Type III in which the paraconal interventricular branch originates independently from the aortic sinus. According to Kazzaz and Shanklin (1950), there are 4 to 11 ventricular branches. Pianetto (1939) found two to six principal ventricular branches. Most of the hearts had five main ventricular branches leaving the circumflex branch, but great variation was found. When the left ventricular branches of the paraconal interventricular branch are well developed, they are a major source of supply to the parietal wall of this chamber, and the ventricular branches of the circumflex branch are confined to a triangular area adjacent to the coronary and subsinuosal interventricular grooves. When the first or first few branches of the circumflex branch cross the superficial muscle fibers of the left ventricle obliquely, they are short; they are much longer when they parallel the superficial muscle fibers as well as the left ventricular branches of the paraconal interventricular vessel. Usually the longest branch leaving the circumflex branch lies adjacent and parallel to the subsinuosal interventricular branch. This is appropriately known as the left marginal branch (ramus marginalis sinister), as it follows the left border ventrally on the surface of the left ventricle. The atrial branches of the circumflex branch of the left coronary artery, which supply the left atrium and auricle, are small and variable. The first branch arises deep to the great coronary vein and, extending dorsally and toward the center of the base of the heart, supplies the deep surface of the left atrium and the ventral part of the interatrial septum. It is the largest of the right atrial branches and, according to Meek et al. (1929) its terminal branches may partly encircle the termination of the cranial vena cava, where they anastomose with the right atrial vessel to the sinoauricular node. It may be the main source of supply to this node. At least two other small branches cross the lateral surface of the great cardiac vein or the coronary sinus and supply the right atrium. The paraconal interventricular branch (ramus interventricularis paraconalis) is approximately the same diameter as the circumflex branch, and averages 1.5 mm in width. It is approximately 7 cm long as it winds obliquely and distally from left to right across the right ventricular border of the heart in the paraconal interventricular groove. It usually extends around the apex of the heart (Christensen, 1962). It has left ventricular, right ventricular, and septal branches. The septal branch (ramus septalis) (Figs. 11-7A and 11-11), as found by Donald and Essex (1954) in the 125 specimens they studied, arose as follows: 1. Paraconal interventricular branch, 48% 2. As one of the three terminal branches of the left coronary artery, 27% Immediately after entering the interventricular septum, the septal branch usually runs obliquely toward the apex of the heart, giving off major and minor branches along its course. Initially it runs obliquely to the right ventricular side of the septum. In this course it may parallel the right atrioventricular fibrous ring as it lies under the endocardium adjacent to the dorsal half of the septal cusp of the right atrioventricular valve. The first half of the artery lies deep to the endocardium of the right ventricle; the second half penetrates deeply into the septum. It supplies all the main papillary muscles of the right ventricle. Wilson and Scheel (1989), using gradual Ameroid occlusion of the circumflex branch, demonstrated that the septal branch is a significant source of intramyocardial collaterals to other coronary branches in young dogs. According to Donald and Essex (1954), the septal branch supplies 70% to 75% of the interventricular septum. These authors and many others have found that the anastomoses between the septal branch and the adjacent arteries of the canine heart are not sufficiently large or numerous to permit retrograde filling of the septal branch with injected dye. The subsinuosal and paraconal interventricular branches supply the periphery of the interventricular septum. The paraconal vessel contributes much more blood than does the subsinuosal vessel (Christensen & Campeti, 1959). Hyde and Buss (1986) examined the transmural microvasculature adjacent to the papillary muscles of the left ventricle to address the question of whether a transmural gradient in components of the coronary microvasculature exists in the dog heart. Their results suggest that transmural differences in coronary blood flow are not due to transmural structural differences but rather to physiologic regulatory mechanisms of coronary blood flow. The cardiac veins (venae cordis), although in many instances satellites of the arteries to the heart, do not take the names of the comparable vessels. Most of the blood to the heart is returned to the right atrium by a short, wide trunk, the coronary sinus. Some of the ventral cardiac veins and the smallest cardiac veins (Thebesian) open into the cavities of the heart directly. Truex and Angulo (1952) demonstrated that the tributaries of the coronary sinus form an extensive venous plexus in the left ventricle and septum, which is predominant compared with corresponding branches of the left coronary artery. They found that the coronary sinus and cardiac veins provided continuity with the capillary bed of the myocardium. The coronary sinus had anastomoses with cardiac veins, myocardial sinuses, and Thebesian vessels that provided outlets to the lumen of the right atrium. Esperanca-Pina et al. (1981) studied 47 dog hearts using an injection-corrosion-fluorescence technique to visualize the superficial cardiac veins draining the coronary sinus. They noted a large number of Thebesian veins in the subendocardium of the interventricular septum (as imagined by Galen) and all of the cardiac chambers except the internal wall of the left atrium. The coronary sinus (sinus coronarius) (Figs. 11-3 and 11-6) is the dilated terminal end of the great coronary vein. It is approximately 2 cm long and 5 to 8 mm in diameter. It lies in the fat of the dorsodextral part of the coronary groove ventral to the caudal vena cava and dorsal to the terminal part of the circumflex branch of the left coronary artery. It opens into the right atrium ventral to the termination of the caudal vena cava. It may be partly covered by a few muscle fibers derived from the left atrium. Piffer et al. (1994) found a valve between the great cardiac vein and the coronary sinus in 4 out of 34 dog hearts they studied. This small, inefficient semilunar valve of the coronary sinus (valvula sinus coronarii) is located at the termination of the coronary sinus. In the 40 dogs studied, Maric et al. (1996) found the tributaries to the coronary sinus highly variable, with the great and middle cardiac veins the main and constantly present tributaries. The great cardiac vein (v. cordis magna) (Figs. 11-3 and 11-4) lies in the dorsal part of the coronary groove as it circles the caudal surface of the heart from the left. It arises near the apex of the heart and ascends toward the base in the paraconal interventricular groove, where it is usually paired. Along its course it collects numerous veins from the ventricles and small twigs from the left atrium. Most of those from the ventricles are paired, in that a vein lies on each side of the comparable artery. At its termination in the coronary sinus the diameter of the passage increases threefold, according to Meek et al. (1929). At this place two veins usually enter the great cardiac vein or the coronary sinus. The larger branch, which may not be paired, ascends from near the apex of the heart and is known as the dorsal vein of the left ventricle (v. dorsalis ventriculi sinistri). It is the largest vein draining this chamber. Frequently a smaller vein, the oblique vein of the left atrium (v. obliqua atrii sinistri) can be seen entering the coronary sinus dorsally after emerging from deep to the pulmonary veins (see Fig. 11-3). Its importance lies in the fact that, like the coronary sinus, it is a vestige of the embryonic left common cardinal vein. It may persist as a left cranial vena cava (Fig. 12-3), or it may be nonpatent. The middle cardiac vein (v. cordis media) (Fig. 11-5) ascends in the subsinuosal interventricular groove in company with or near the subsinuosal interventricular artery. It is a large paired vessel that collects tributaries from both ventricles and empties into the coronary sinus near its termination. Near the apex of the heart, where the subsinuosal and paraconal interventricular grooves merge, the middle and great coronary veins sometimes anastomose. Thebesian veins are microscopic channels of venule size that open into every chamber of the heart and, within the myocardium, anastomose with both the coronary artery branches and other cardiac veins. It is possible that when gradually occluding lesions develop, the blood may flow in a retrograde direction in these valveless veins, which hypertrophy, to provide nourishment for the affected part. Pina et al. (1975) studied the Thebesian veins of the heart in 48 dogs using a corrosion fluorescence method. They found five types of terminations: arborform, sinuous, brushlike, canaliculated, and stellate. Although these small cardiac veins are found in the walls of all chambers of the heart, they are most often found in the right ventricle (81% of dogs) and right atrium (77% of dogs). The pulmonary trunk (truncus pulmonalis) (Figs. 11-2, 11-3, 11-4, 11-9, and 11-10) and its branches are the only arteries in the body that carry unaerated venous blood. The trunk arises from the pulmonary fibrous ring at the conus arteriosus and, after a course of approximately 4 cm, it divides into the right and left pulmonary arteries. At the conus arteriosus the trunk is flanked by the right and left auricles. Farther out on the trunk, but still within the fibrous pericardium, fat usually masks the true length and position of the intrapericardial part of the vessel. The cranial, lateral, and caudal surfaces of the proximal three fourths of the vessel are covered by serous pericardium and fat. The distal fourth of the vessel serves for the attachment of the fibrous pericardium and can be examined without opening the pericardial cavity. The pulmonary trunk, along its entire medial surface, contacts the aorta. The two vessels form a slight spiral as they obliquely cross each other. The ligamentum arteriosum (Fig. 11-3) is a connective tissue remnant of the fetal ductus arteriosus, which arises from the pulmonary trunk near the bifurcation of the pulmonary trunk and passes to the aorta. Everett and Johnson (1951) studied the closure of the ductus arteriosus in the dog and found that it remains patent, to a variable degree, for a considerable time after birth. Some reduction in flow occurred 1 to 2 hours after birth, and more marked reduction occurred at 9 hours postpartum. Anatomic obliteration of the slitlike opening was complete at 15 to 18 days postpartum. House and Ederstrom (1968) found that the ductus arteriosus was open in all puppies at less than 4 days of age and did not close anatomically until they were 7 or 8 days of age. For radiographic aspects of patent ductus, see Buchanan (1972). A latex cast of a patent ductus arteriosus in an adult dog is shown in Figure 11-9B. The right pulmonary artery (a. pulmonalis dextra) (Figs. 11-3, 11-5, and 11-6) is approximately 2 cm long and 1 cm in diameter. It leaves the pulmonary trunk at nearly a right angle and runs to the right, where at first it is in contact with the concavity of the arch of the aorta and later with the right bronchus that is dorsal to the artery. It lies obliquely across the base of the heart between the cranial and caudal venae cavae. Its first lobar branch enters the right cranial lobe of the lung, right cranial lobar branch (ramus lobi cranialis). Approximately 1 cm distal to the origin of this branch the vessel divides into a middle lobar branch (ramus lobi medii) and a caudal lobar branch (ramus lobi caudalis) that provide numerous vessels that supply the middle, caudal, and accessory lobes of the right lung. The left pulmonary artery (a. pulmonalis sinistra) (Figs. 11-3 and11-4) is shorter and slightly smaller in diameter than the right artery. It is partly covered dorsally at its origin by the left principal bronchus. The artery then passes obliquely across the pulmonary vein coming from the cranial lobe and divides unevenly into two or more branches. The smaller branch or branches enter the cranial part of the cranial lobe, left cranial lobar branch (rami lobi cranialis). The large caudal lobar branch (rami lobi caudalis) enters the bulk of the left lung, where it subdivides and supplies the caudal part of the cranial lobe and the caudal lobe of the lung. The relations of the pulmonary arteries within the lungs are described in Chapter 8, Respiratory System. The pulmonary veins (vv. pulmonales) are valveless and return aerated blood from the lungs to the left atrium. Unlike the pulmonary arteries, the pulmonary veins from each of the lobes of the lungs as a rule retain their separate identity to the heart. An exception to this is frequently found in the veins from the right caudal and accessory lobes, which fuse just before entering the atrium. Often, the vein from the left caudal lobe joins the venous trunk from the right side that results in a single opening in the left atrium, thus serving for the return of the blood from the right and left caudal and accessory lobes. Other variations are common. Frequently, two veins may enter the heart directly from one of the lobes. Usually, however, several veins converge and empty into the heart by a single vessel, which ranges between a few millimeters to approximately 1.5 cm in length. In medium-sized dogs, all pulmonary veins are more than 5 mm in diameter as they enter the heart. The pulmonary veins may be divided into the right pulmonary veins (vv. pulmonales dextrae) from the right lung, and the left pulmonary veins (vv. pulmonales sinistrae) from the left lung. The pulmonary veins within the lungs are described with the intrinsic structure of the lungs in Chapter 8. The aorta leaves the left ventricle near the center of the base of the heart (Fig.11-7A). It is a thick-walled vessel through which all the systemic blood of the body passes. All of the large systemic arteries arise directly from it. For descriptive purposes, it may be divided into an ascending and a descending portion, separated by the aortic arch. The initial part attaches to the fibrous base of the heart, is largely located within the pericardium, and is known as the ascending aorta (aorta ascendens). It is approximately 2 cm long before it makes a U-turn dorsocaudally and to the left as the aortic arch (arcus aortae). The remainder of the aorta, from the arch to its terminal iliac branches, is the descending aorta (aorta descendens). The descending aorta may be divided further into a thoracic part (aorta thoracica) and an abdominal part (aorta abdominalis). The normal development of the heart and aortic arches in several domestic animals is discussed by Krediet (1962) in a thesis on anomalies of the arterial trunks in the thorax. The brachiocephalic trunk (truncus brachiocephalicus) (Fig. 11-12), the first large artery from the aortic arch, passes obliquely to the right and cranially across the ventral surface of the trachea. It is approximately 4 cm long and 8 mm in diameter. The left common carotid artery is the first branch to leave the brachiocephalic trunk. Frequently, a small branch leaves the brachiocephalic trunk close to the heart to aid in the supply of the thymus and pericardium. Jarvis and Nell (1963) have described various origins of a tracheoesophageal branch of the brachycephalic trunk and a less frequent thymopericardial branch that have caused difficulties in experimental surgery of the great vessels and esophagus. The brachiocephalic trunk terminates in the right common carotid and the right subclavian arteries. This termination is medial to the first rib or first intercostal space of the right side. The common carotid arteries (aa. carotides communes) (Figs. 11-12 through 11-14) arise from the brachiocephalic trunk approximately 1 cm apart. Of 123 dogs examined, the right and left common carotids arose from the brachiocephalic trunk by a common trunk in four specimens. The interval between the origins of the common carotids varies from 15 to less than 1 mm. When a bicarotid trunk (truncus bicaroticus) is formed, it usually arises from the brachiocephalic trunk approximately 2 cm distal to its origin from the aorta opposite the first intercostal space or second rib.
The Heart and Arteries
Pericardium and Heart
Pericardium
Heart

Size and Weight
Surface Topography
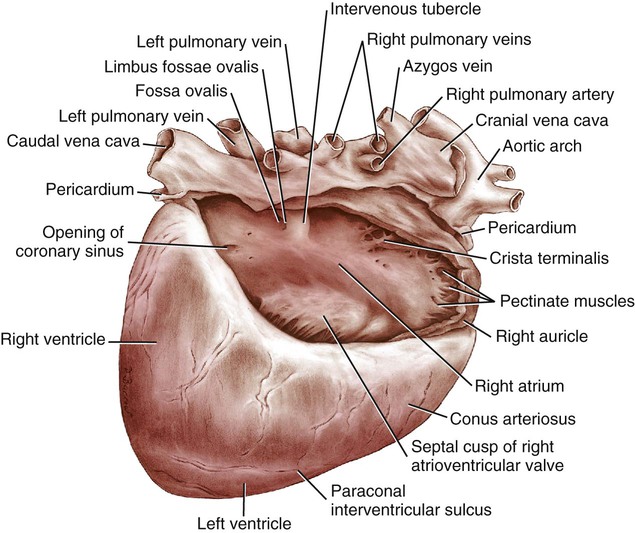
Atria
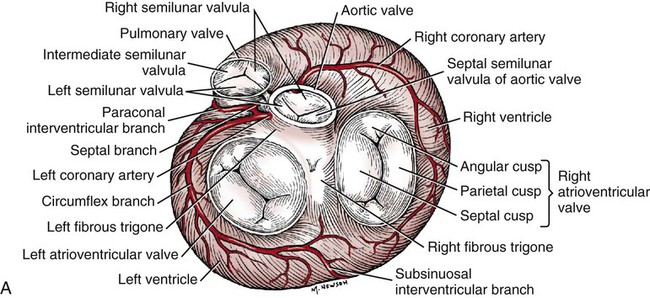
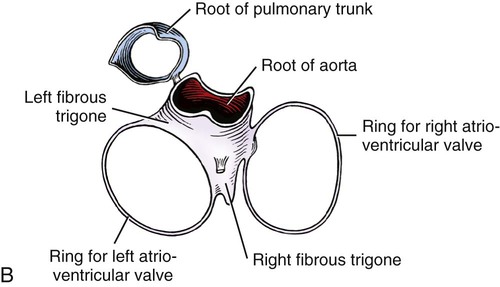
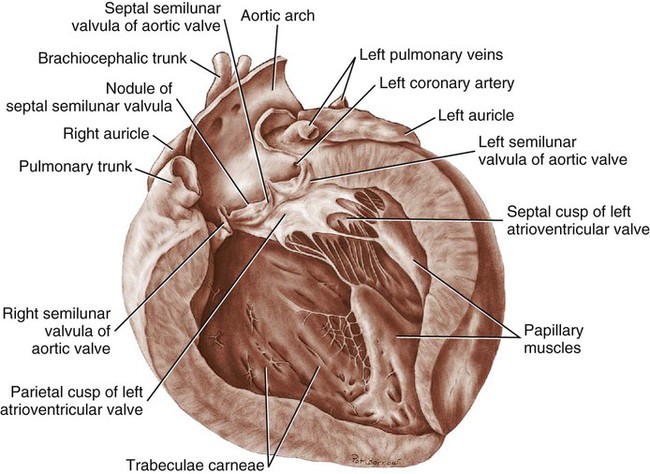
Fibrous Base
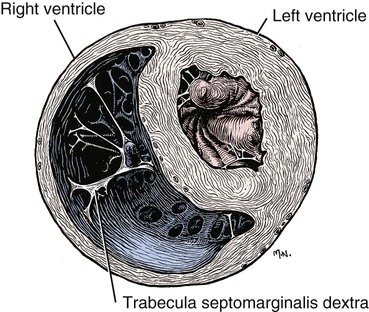
Ventricles
Myocardium
Atrioventricular Valves
Aortic and Pulmonary Valves
Innervation and Conduction System
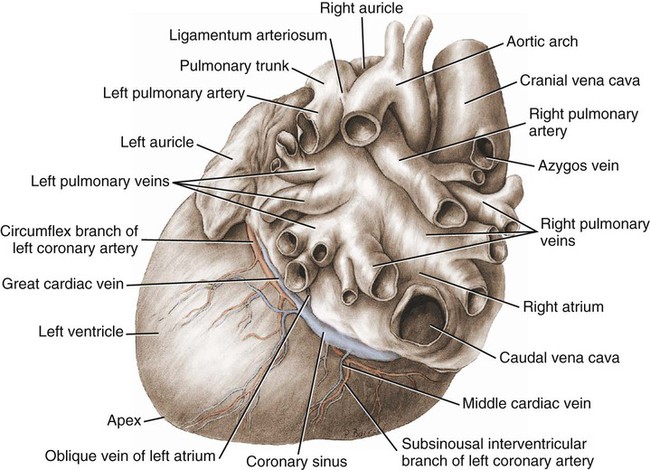
Blood Vessels of the Heart
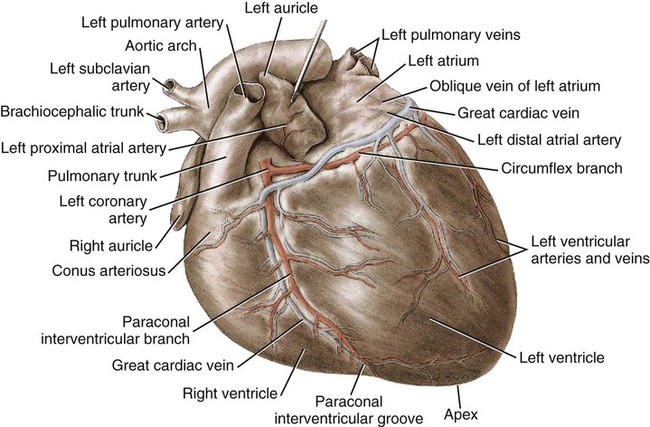
Coronary Arteries
Cardiac Veins
Thebesian Veins
Pulmonary Arteries and Veins
Pulmonary Trunk
Pulmonary Veins
Systemic Arteries
Aorta
Aortic Arch
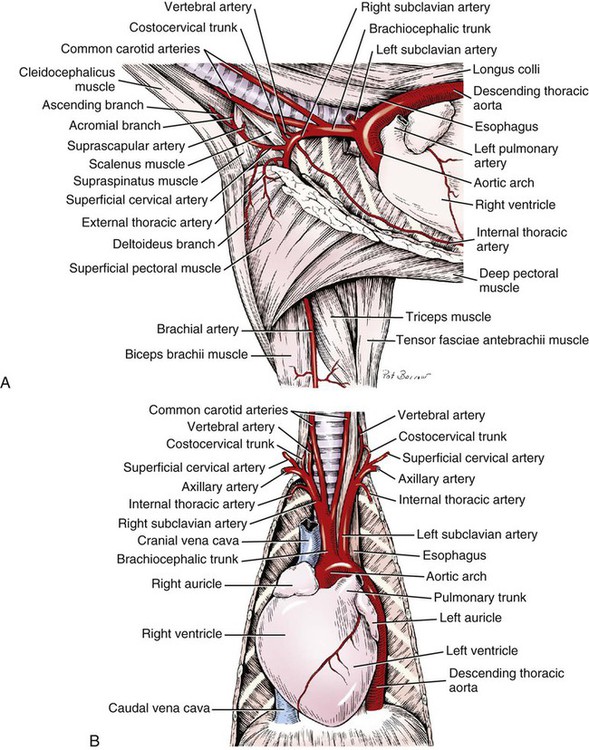
Arteries of the Head, Neck, and Thorax
Brachiocephalic Trunk
Common Carotid Arteries (Box 11-1)