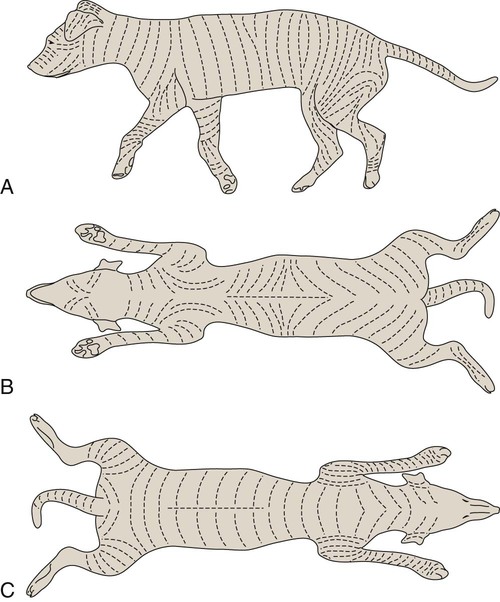
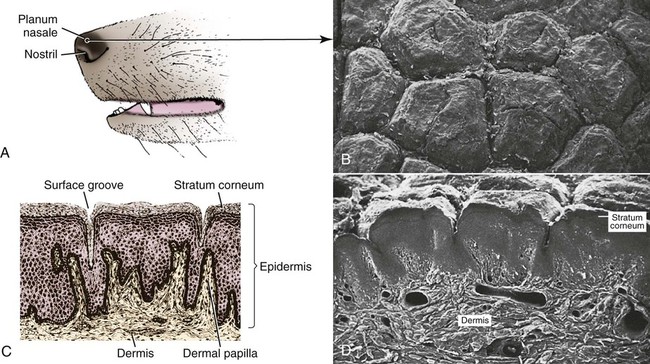
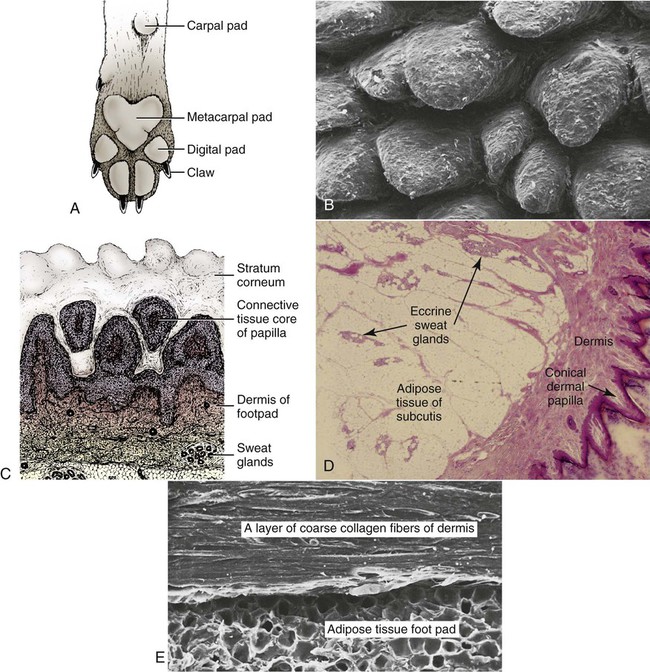
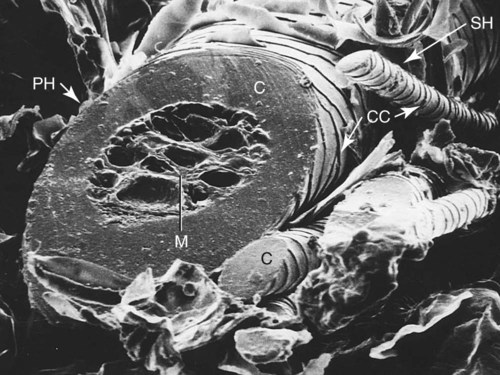
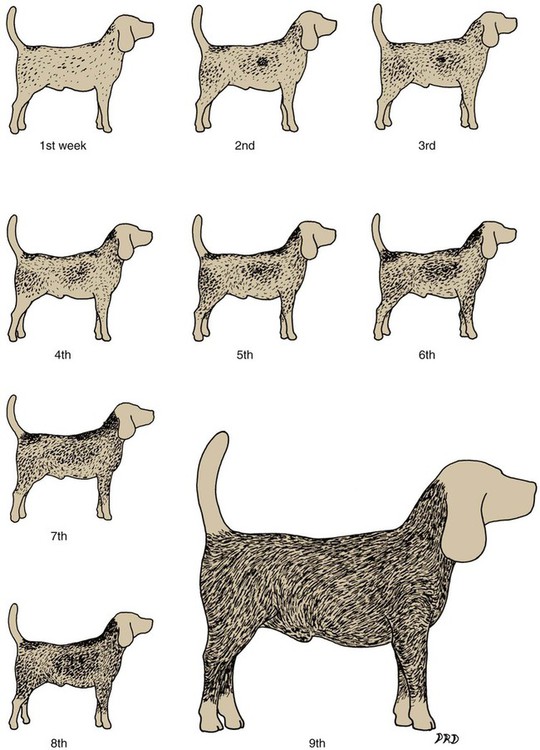
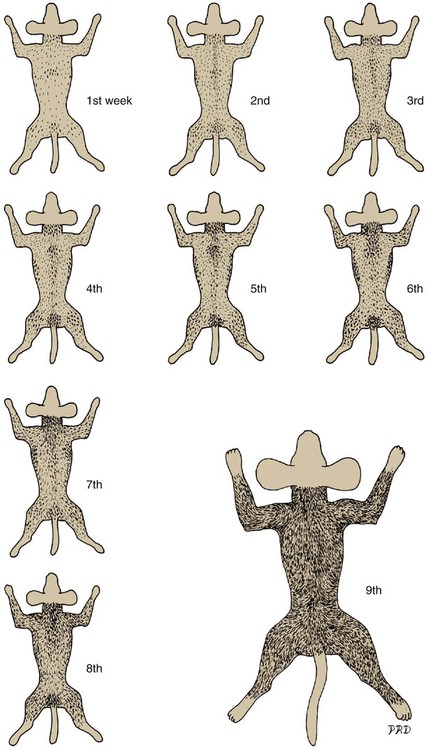
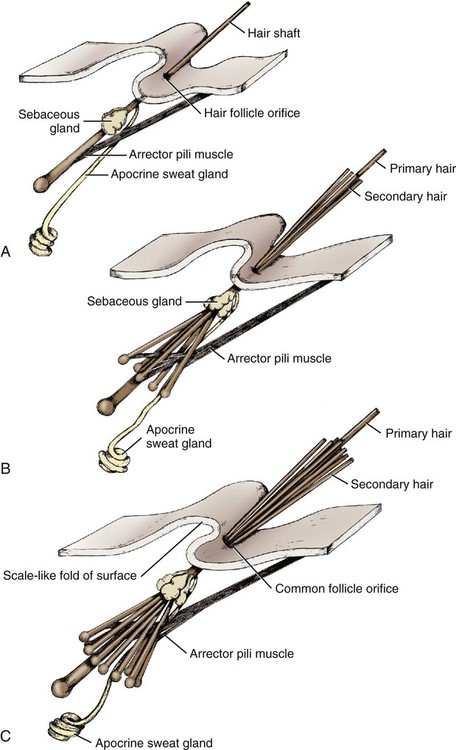
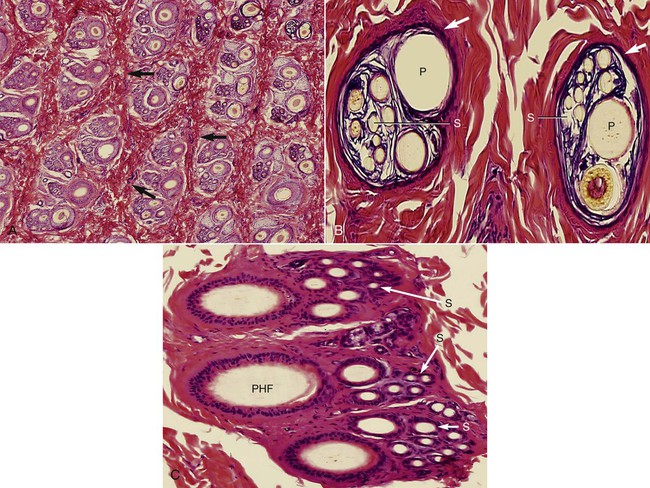
The common integument (integumentum commune) comprises the skin, hair, claws, pads, and skin glands, including the glands of the paranal sinus and the mammary glands (Bereiter-Hahn et al. 1986). The skin (cutis) consists of a superficial epidermis of stratified squamous epithelium, and an underlying connective tissue, the dermis. The interface between the epidermis and the dermis is formed by a functional basement membrane made up of matrix proteins (Ghohestani, Rouselle, & Uitt, 2000). The skin is underlain by a subcutis (tela subcutanea or hypodermis), which is not part of the skin. The subcutis functions as a moveable support for the skin allowing it to glide over underlying tissues. It connects the dermis with the fascia and the various forms of hair (pili) that compose the coat. The skin prevents desiccation and informs the central nervous system of its contacts. The skin of puppies is more permeable. As a sensory organ, the skin is the receptor for the perception of touch, pressure, vibration, tension, noxious stimuli, heat, cold, and harmful chemicals (Iggo, 1962, 1977). It prevents trauma, protects the body from the invasion of microorganisms and noxious chemicals, and regulates temperature change. In regard to heat regulation, however, the skin of the dog serves only a limited role via sweat glands (Iwabuchi, 1967) and superficial capillary beds because of the alternative route of thermal panting (Blatt et al., 1972). The skin lacks superficial arteriovenous shunts (Thoday & Friedman, 1986), and the hairy skin is devoid of eccrine sweat glands (Muller et al., 1989). The skin acts as the site of vitamin D synthesis, and the subcutaneous tissues serve as a reservoir for fat, electrolytes, water, carbohydrates, and proteins. Secretions of skin glands not only waterproof and lubricate the skin, but also function as pheromones for recognition (Parks & Bruce, 1961) and, in the case of the mammary glands, as nourishment for the young. The skin has an immunosurveillance potential and is often subject to allergic reactions, dermatitis, and parasitic invasion. Immunologic events are modulated by the production of a cytosine, an epidermal cell–derived thymocyte-activating factor (Choi & Sander, 1986). The skin may reflect the state of health of the animal as well as indicate cutaneous manifestations of internal disease, such as icterus, cyanosis, and edema. After injury or surgery the edges of skin wounds gape, owing to continuous tension of the skin. There are large tactile hairs (pili tactiles) on the muzzle (pili tactiles labiales superiores), mandible (pili tactiles mentalis) and dorsal to the eyes (pili tactiles supraorbitales). There are usually two genal tubercles on each side of the face, from which long hairs grow. The specialized hairs of the eyelids, or eyelashes (cilia), are stiff and larger than other hairs. The ventral body surface (Fig. 3-1) is characterized by hairless areas, such as the median raphe of the linea alba, the umbilicus, nipples, and the sparsely haired mammary glands (glandula mammaria). The hairy skin is thickest over the neck, dorsal thorax, rump, and base of the tail. The skin of the pinna of the ear, axilla, and inguinal and perianal regions is thinnest. All skin areas are composed of epidermis and dermis (corium) and are underlined by the subcutis (hypodermis, tela subcutanea). The skin is thicker on the dorsal part of the neck, trunk, and tail than on the belly, the flank, or medial side of the limbs. The skin, hair, and subcutis of the newborn puppy represent approximately 24% of the total body weight. Owing to differential growth of various body parts, this percentage is reduced 12% by 6 months of age. Additional information concerning canine skin may be found in Webb and Calhoun (1954), Lovell and Getty (1957), Blackburn (1965), Warner and McFarland (1970), Calhoun and Stinson (1976), Sokolov (1982), Muller et al. (1989), Scott et al. (2001), and Dyce et al. (2002). The thickness of the hairy skin ranges from 0.5 to 5 mm (Scott, 1980). Processing skin samples obtained from dogs for histologic evaluation caused changes in sample dimensions; the samples decreased in length and width 32% and increased in thickness 75%, compared with their original dimensions (Reiner et al., 2005). The thicker portions of the skin are found near the hair follicle orifices and the hairy margins of the mucocutaneous junctions. According to Lloyd and Garthwaite (1982) the canine stratum corneum has a mean thickness of 13 µm and consists of 50 cell layers. The epidermis of nonhairy skin varies in thickness owing to the system of ridges that occur at the dermal-epidermal junction. The nonhairy margins are of the lip, eyelid, prepuce, vulva, and anus. The thickest epidermis occurs on the nasal skin and digital and metapodial pads. The epidermis of the planum nasale is 200 µm at the time of birth and 600 µm at 6 months of age. The epidermis of the foot pads is 200 µm at birth and increases to 1800 µm at 6 months of age. The thickness of various cutaneous sites in the live dog in relation to hydration status and fluid distribution has been recorded by the use of high frequency ultrasonography. The skin thickness (epidermis plus dermis) ranged from 2.211 mm to 3.249 mm. The greatest thickness is in the sacral, frontal, flank and metatarsal regions (in decreasing order). Ultrasonography is a noninvasive tool in the evaluation of skin hydration in healthy dogs and in dogs with skin edema (Diana et al., 2008) and can be used instead of the current evaluation of skin hydration in dogs, which relies on clinical palpation by picking up the skin with the fingers (Chesney, 1995; Hester et al., 2004; Welzel et al., 2001). The greatest skin thickness is found in the Shar Pei breed and the least skin thickness in the Zwergpinscher and the Toy Poodle breeds. There was no correlation between body weight and skin thickness of dogs according to Diana et al. (2004). A positive correlation in vivo was detected between cutaneous thickness measured by the use of ultrasonography and measurements obtained by the use of histologic examination, which is invasive, time consuming, and costly (Diana et al., 2004). The thickness of the dermis, or corium, varies in different body areas and at different ages. The dermis of the planum nasale and foot pads is 300 µm at birth and increases to 800 µm at 6 months of age. The differences in thickness of the hairy skin that can be observed by comparing the dorsal area with the abdominal area are due to the difference in thickness of the dermis. Dermal papillae of the hairy skin are not present in the dog owing to the lack of the epidermal rete ridges (Muller et al., 1989). The dermis of the skin of the dorsal region is 700 µm at birth and increases to 1500 µm at 6 months of age. The dermis in the abdominal region is 300 µm at birth and increases to 800 µm at 6 months of age. The dermis is composed of fibroblasts, fibers, and various structures such as blood vessels, nerves, cells of blood or tissue origin, and tissue fluid. According to Muller et al. (1989), the dermis of the Chinese Shar-Pei, a breed with excessive skin folds, contains a considerable amount of mucin. Fibronectin released by fibroblasts, endothelial cells, and histiocytes (Clark, 1983; Quaissi & Capron, 1985) regulates vascular permeability, wound healing, and cytoskeletal orientation (Muller et al., 1989). Skin glands and hair follicles are embedded in the dermal tissue. Fibers of smaller diameter are found in the superficial dermis adjacent to the epidermis. The larger collagenous fibers are in the deeper layer of the dermis. The size of the fibers, the population density of fibroblast nuclei, and the plasma cell content of the dermis undergo developmental changes with the increase in thickness that occurs between birth and 6 months of age. At the time of birth, there are many reticular fibers throughout the dermis. By 3 weeks most of them have been replaced by collagen fibers. A few reticular fibers remain just deep to the epidermis, around hair follicles, and surrounding sebaceous and sweat glands. Collagen fiber bundles increase in size and number as the dermis thickens with age. Collagen fibers account for 90% of the fiber component of the dermis (Thomsett, 1986). At birth, the collagen fiber bundles measure 3 or 4 µm in diameter, and at 6 months they measure 19 to 20 µm. Concurrent with this gradual increase is a corresponding decrease in the size of the spaces between the fiber bundles. There is also an increase in the size and number of elastic fibers. At birth, elastic fibers are small, branching, and filamentous, less than 0.5 µm in diameter. By 6 months they are thick, undulating fibers of 1.5 to 2 µm in diameter. Irwin (1966) illustrated tension lines in dog skin that are determined by the orientation of connective tissue, gravity, and physical forces. Senile changes in dog skin were noted by Baker (1967). A skin incision made across the tension line gaps maximum, heals in longer time, requires more sutures, and results in a wider scar. A skin incision made parallel to the tension lines requires fewer sutures and heals better with minimum or no scar (Fig. 3-2). Fibroblast nuclei in the dermis are more densely distributed at birth than at 6 months of age. Per unit area, there are twice as many nuclei at birth as at 6 months. Mast cells occur in all parts of the dermis and subcutaneous fat tissue (Emerson and Cross, 1965). They are present in greatest numbers in the reticular layer of the dermis, with fewer in the papillary layer and subcutis. They are usually most numerous around the walls of small blood vessels (Sokolov, 1982) and frequently surround sebaceous glands and apocrine or eccrine glands. They are most numerous in the skin of the ears and appear in decreasing numbers in the skin of the vulva, prepuce, medial thigh, foot pad, and external nares. The subcutis consists of panniculus adiposus and fibrous connective tissue. The subcutis anchors the dermis to the bone periosteum, muscle perimysium, and cartilage perichondrium. Subcutis fat is characteristic of the carpal, metacarpal, metatarsal, and digital pads; it acts as a shock absorber. The adipose layer varies according to breed and individual variations for body regions (Schwarz et al., 1979). The histologic examination of various regions of aged dogs showed atrophy of the epidermis, appendages, and dermis with decrease in hair numbers (Baker, 2008). Conroy and Beamer (1970) studied the development of melanoblasts and melanocytes in the skin of Labrador Retriever fetuses. The earliest melanoblasts were demonstrable in the primordial dermis of the head, thorax, and abdomen of 29-day fetuses. The melanoblasts were most numerous in the deeper two thirds of the primordial dermis in contact with or near blood vessels. The frequent contact or close association of melanoblasts with blood vessels suggests that the cells migrate along blood vessels in their journey from the neural crest to their destination in the epidermis. Dendritic dermal melanocytes first appeared in the 37-day fetus, and scattered epidermal melanocytes appeared in 29-day fetuses. Their numerical distribution in various regions of the body conformed to a dorsoventral gradient. Melanocytes were present in primordial and differentiating hair follicles, eccrine sweat glands, and sebaceous glands. The nasal skin is usually heavily pigmented, tough, and moist. On close examination of the surface of the planum nasale, polygonal plaquelike areas are observed, which give the nasal skin an irregular appearance (Fig. 3-3A and B). On histologic examination, no glands can be demonstrated in the epidermis or dermis of nasal skin. The moisture that appears on the nasal surface is derived primarily from serous gland secretions of the lateral nasal gland and other glands that drain into the vestibule (Blatt et al., 1972). The dermis of nasal skin is composed of reticular, collagenous, and elastic fibers, together with fibroblasts, blood vessels, and nerves. The blood vessels and nerves are larger in the deeper layers of the dermis than in the more superficial layers. Adjacent to the epidermis, the dermal papillae interdigitate with epidermal projections to form an irregular line of attachment between the dermis and epidermis (Fig. 3-3C and D). The epidermis of the nasal skin, which averages 630 µm in thickness in adult dogs, is composed of three layers: stratum basale, stratum spinosum, and stratum corneum. The stratum basale of the epidermis rests on a basement membrane underlined by the condensed thickened superficial portion of the dermis and consists of one layer of cylindrical cells. The stratum spinosum is made up of 10 to 20 layers of diamond-shaped, dome-shaped, or flattened polygonal cells that have a lighter-staining cytoplasm than that of the cylindrical cells. In heavily pigmented nasal skin there are many pigment granules in the cytoplasm. There is no stratum granulosum or stratum lucidum in the epidermis of the nasal skin. The more peripheral spinosum cells apparently do not undergo keratinization, as they do in other regions of epidermis. Their cytoplasm becomes weakly acidophilic and the nuclei become pyknotic with the cells flattening out into a squamous type. As they approach the surface, they remain as a thin, atypical nucleated stratum corneum, four to eight cell layers thick. Nose prints, similar to finger prints, can be used to distinguish between individuals (Horning et al., 1926). The skin of the digital pads, torus digitalis, is usually heavily pigmented and is the thickest region of canine skin. The surface of the pads is smooth in cats and rough in dogs, owing to the presence of numerous conical projections that are heavily keratinized and are readily seen with the naked eye (Fig. 3-4A and B). When dogs are kept on concrete or rough surfaces, the projections sometimes become worn smooth so that they are rounded instead of conical in shape. The digital cushion, or base of the foot pad, is made up of subcutaneous adipose tissue that is partitioned by reticular, collagenous, and elastic fibers. Many elastic fibers are present in the deeper layers. Eccrine sweat glands and lamellar corpuscles are embedded in the adipose tissue (Fig. 3-4C and D). The long excretory ducts of the eccrine glands are found deep in the dermis, through which they carry secretion to the surface of the epidermis. Adjacent to the epidermis, the dermal connective tissue is dense and papillate, forming conical dermal cores for the epidermis. There are also secondary dermal papillae within the conical structure. The epidermis of the digital pad, which averages 1800 µm in thickness in the adult dog, is composed of five layers: stratum basale, stratum spinosum, stratum granulosum, stratum lucidum, and stratum corneum. The stratum basale is made up of a single layer of basal cells resting on the basement membrane. The stratum spinosum is composed of 10 to 15 layers of diamond- or dome-shaped cells. In both the digital pads and the planum nasale, cell outlines and intercellular bridges (desmosomal attachments) may be observed on the spinous cells. The stratum granulosum is made up of four to seven layers of flattened cells that contain basophilic keratohyalin granules in their cytoplasm. The stratum lucidum is a completely keratinized layer of dead cells (Scott, 1980) and appears as a shiny, acidophilic layer of homogenous substance with refractile droplets called eleidin. The stratum corneum of the digital pads consists of a thick layer of keratinized nonnucleated material thicker than all the cellular layers combined. The excretory ducts of the eccrine sweat glands of the digital pad become continuous with the epidermis, where their epithelium joins with the stratum basale of the epidermis. The lumen of the excretory duct follows a tortuous path through the epidermal cells to the surface, where the glandular secretion is expelled. The basic unit of hair production is the individual hair follicle (folliculus pili). The follicle wall, which is continuous with the surface epithelium, is divided into two layers, the external and internal root sheaths. The follicle attains its greatest diameter at the base, where it is dilated to form a bulb, in which the hair-producing matrix is contained. Invaginating the bulb is the dermal papilla, which supplies the germinative epithelium by diffusion as long as the hair is growing. The hair shaft (Fig. 3-5) consists of a central medulla; a thick cortex, which forms the bulk of the hair; and a single-layered cuticle on the outside. The keratin shaft of the hair is formed by the germinative epithelium of the bulb region, which is active only during the time of hair growth. It has been reported that immunohistochemical evaluation indicated that the bulge-like (hair bulb) region of the dog hair follicle contains stem cells (Mercati et al., 2008). There are periods during which the growth of the hair is arrested. At this time there is a regression of the hair root, and the dead club hair is held in the follicle completely disconnected from the inactive germinal matrix. After a variable period, the dormant germinal cells become active and enter a period of organogenesis in which a new hair root is regenerated, and production of hair is resumed. At this time the old dead hair will be shed and replaced by the new hair. Growing hair follicles are said to be in anagen, and quiescent ones in telogen; the period of transition between the two is called catagen. Differences may be observed in hair growth rates in various breeds and during certain seasons of the year. Al-Bagdadi (1975) found that the average rate of daily hair growth in male Beagle dogs was 0.4 mm/day in the winter and 0.34 mm/day in the summer. Butler and Wright (1981) reported determinations from male Greyhounds to be 0.04 mm/day in the summer and 0.18 mm/day in the fall. Although the two observers found widely different values, which may reflect strain differences, they agreed that the daily growth rate of the hair shaft was greater during the colder season than it was during the warmer time of the year. The rate of hair growth in mongrel dogs varies individually and by region of the body (Gunaratnam & Wilkinson, 1983). The pattern of regrowth of the clipped hair coat of a Beagle dog is illustrated in Figs. 3-6 and 3-7 (Al-Bagdadi, 1975). According to Diaz et al. (2006), the hairs in the lumbosacral region of Siberian Husky breed were proportionally shorter than the lateral thigh hairs 2 months after clipping. Brushing had no effect on hair regrowth after clipping the normal dog. The terms pregerm, hair germ, hair peg, and bulbous peg are used to designate developmental stages of the canine hair follicle. In a study of the development of cutaneous pigment, Conroy and Beamer (1970) described the embryologic development of the canine hair follicle. The first evidence of a follicle in the embryo is seen as a thickening of the epidermis (pregerm stage). The pregerm stage passes rapidly into the hair-germ stage as the basal cells become taller and the entire structure sinks into the dermis. From its point of origin the hair germ grows obliquely deep into the mesenchyme in the form of a solid column. This is called the hair-peg stage. The advancing border enlarges, becomes bulbous, and envelops part of the mesenchymal material ahead of it, thus entering the bulbous-peg stage. Later the hair bulb and the dermal papilla become differentiated into the productive hair follicle complete with glandular and muscular accessories. As the dermis increases in thickness between birth and 6 months of age, the length of the hair follicles increases. In 40-day fetuses, all stages of follicles have developed. Secondary hair follicles (Fig. 3-8) begin development before birth but usually have no external hair shaft until after birth. The first hairs to appear in the 29-day fetal dog are in the region of the eyelids, superior lip, and chin (see Chapter 2). These develop into large tactile hairs of the face, which will become specialized sinus hairs. The follicles of the general hairy skin appear in the pregerm stage on the head and neck as early as 30 days. They reach the hair-germ stage at 32 days and hair-peg stage at 37 days of gestation. In the general development of the pelage, the hairs are farthest advanced near the head, and development spreads caudally and ventrally. The primary hair germs form more or less simultaneously at fairly even distances. As the skin grows, increasing the surface area, new primary germs develop among the earlier ones. This results in two, three, or four groups of follicles being clustered together. Later, the secondary germs develop close to the primary ones and form the complex follicle arrangement. This process starts before birth and is completed after birth (see Fig. 3-8). The embryologic development of hair was described by Pinkus (1958). Observations on the development of the hair follicle in the dog have been reported by Al-Bagdadi et al. (1977b). It can be observed by examining the hair coat of a puppy during the first few days after birth when there is usually only a single hair emerging from each external follicle orifice of the skin. On microscopic examination it can be observed that secondary follicles form as strands of intensely basophilic cells running deeply into the dermis from their point of origin adjacent to the sebaceous gland of the primary follicle. These satellite, or accessory, hairs appear externally at 3 or 4 weeks of age, when each primary follicle can be seen to be giving rise to two or three secondary hairs. At 8 to 10 weeks of age the secondary follicles are arranged in a crescent around the central and lateral primary hairs. They are on the same side as the apocrine gland. Subsequently, secondary follicle formation continues until puberty, when from 6 to 10 or more hairs may emerge from a single follicle orifice. The larger primary hairs have a well-developed honeycomb-like medulla (Al-Bagdadi et al., 1988). Their nerve and blood supply is better developed than that of the secondary hair follicles. As a general rule, the coarser guard hairs appear earlier than these secondary hairs. In the development of the dog, the nature of the puppy hair changes, and in the young adult, the fine, fluffy hair of the pup is replaced by a coarser hair. In a young adult dog, the hair growth is profuse and abundant. In old dogs the hair covering is thinner, the hairs are not as long, and frequently the coloring fades to gray. As the hair becomes more brittle, flexibility of the skin and subcutaneous tissue decreases. The hairy skin of an adult dog contains complex hair follicles that are bundles of hairs that share common openings on the surface. These complex hair follicles are usually arranged in groups of two or three oriented in rows. The typical complex group consists of a group of secondary, or underhairs, and a single longer and stiffer primary or cover hair. The primary hair of a three-bundle group is coarser than the thinner secondary hairs (Fig. 3-9).
The Integument
Epidermis
Dermis
Structure of the Dermis and Changes with Age
Pigmentation
Nasal Skin
Digital Pads
Hairy Skin
Growth Rate of the Hair Shaft
Embryology of Hair Follicles
Development of Complex Follicle
Complex Hair Follicle
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


The Integument
Only gold members can continue reading. Log In or Register to continue