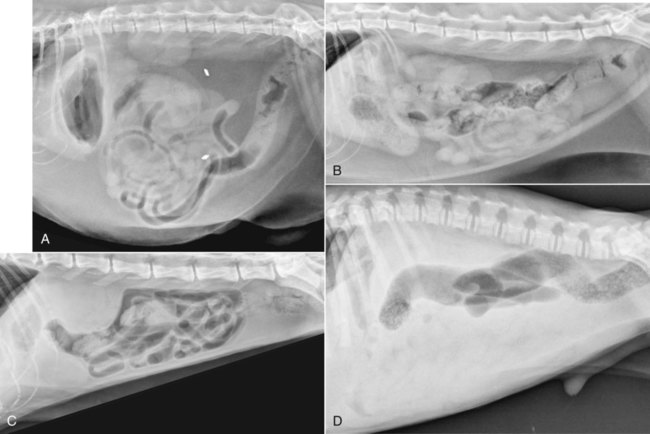
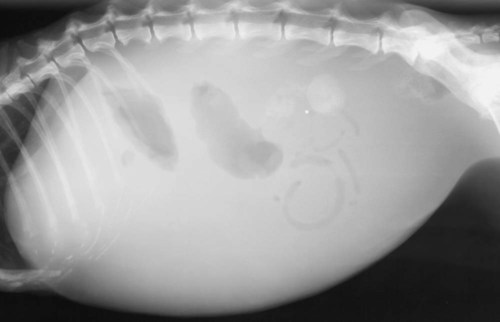
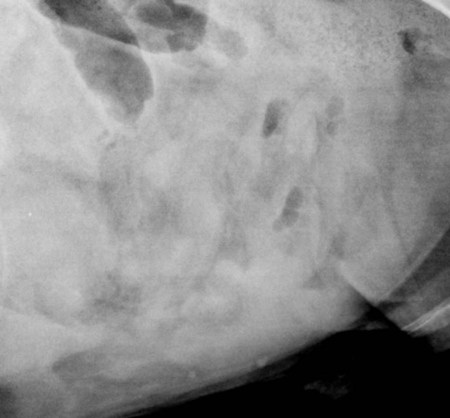
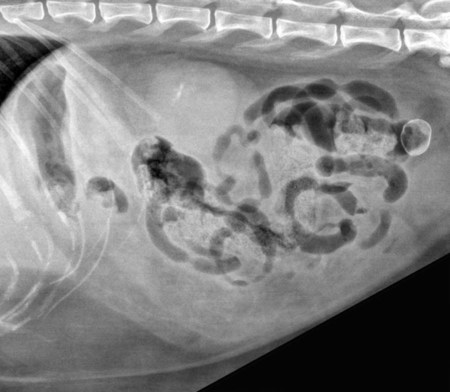
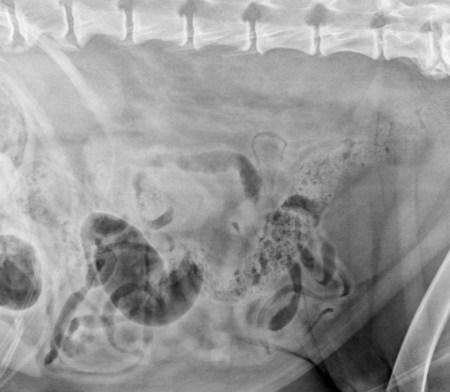
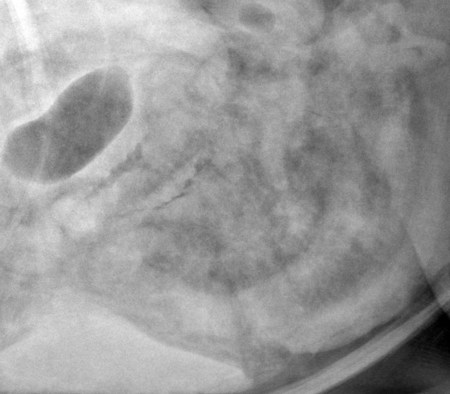
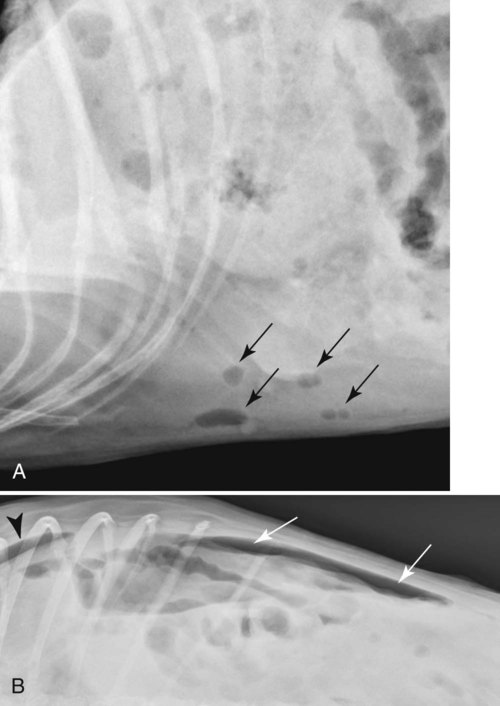
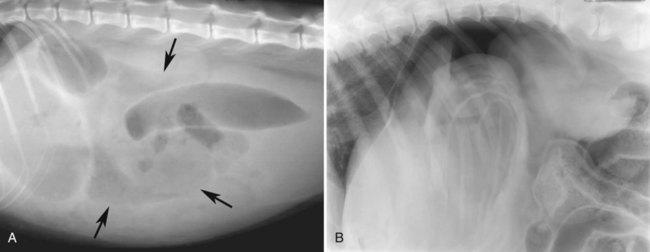
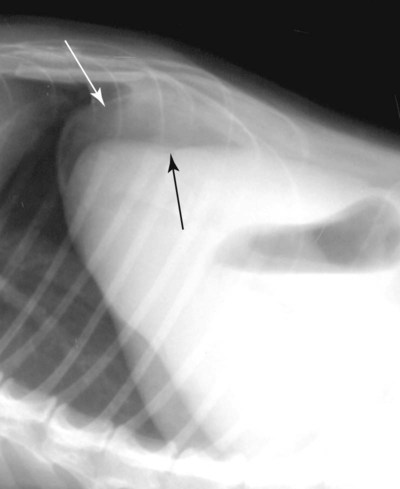
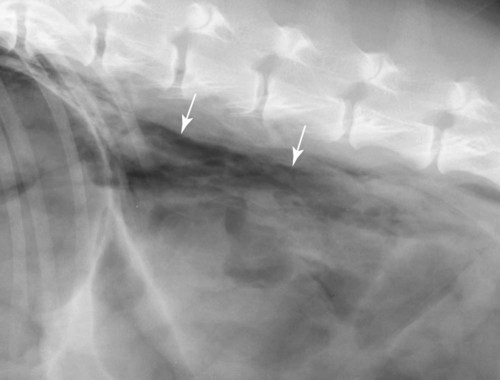
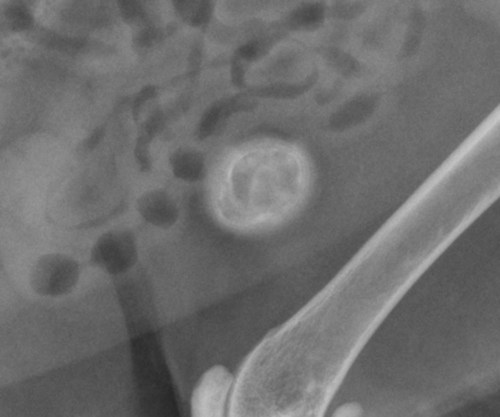
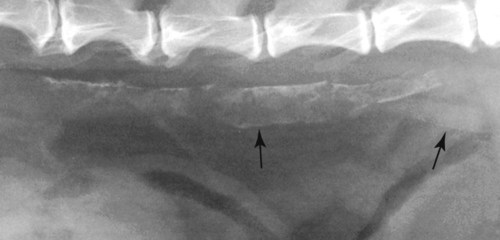
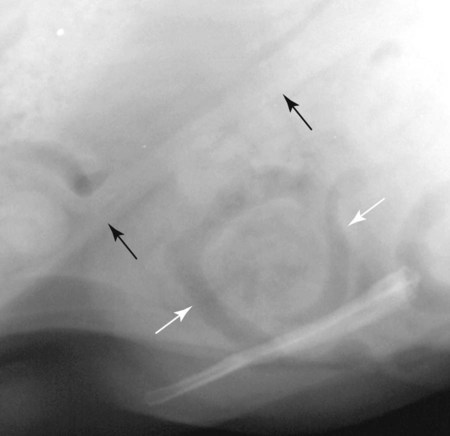
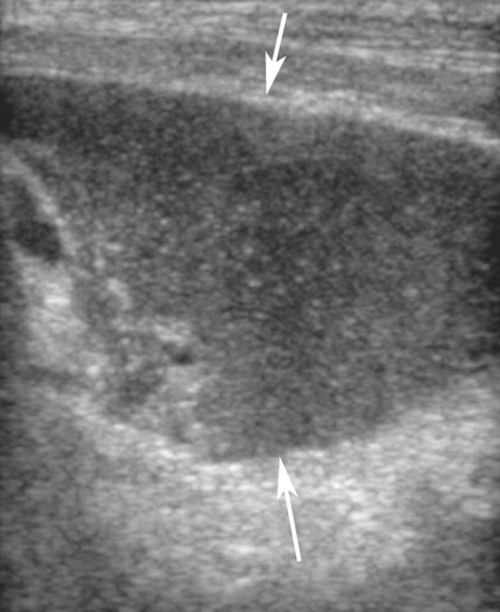
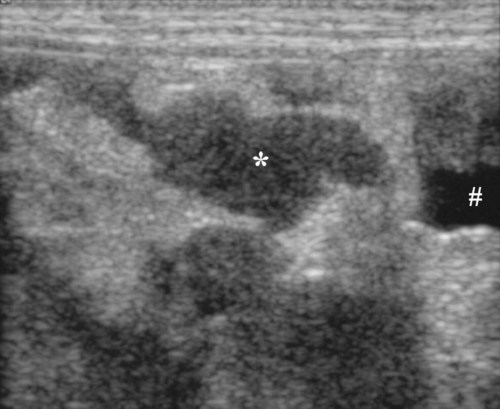
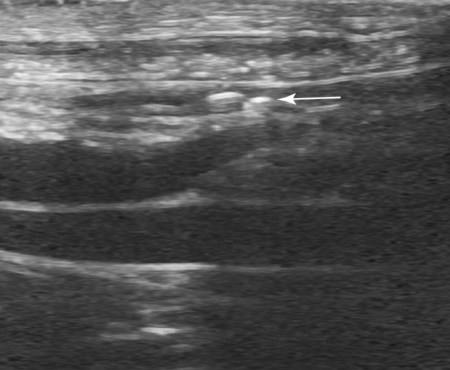
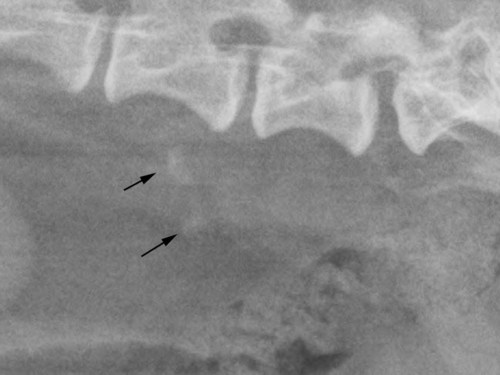
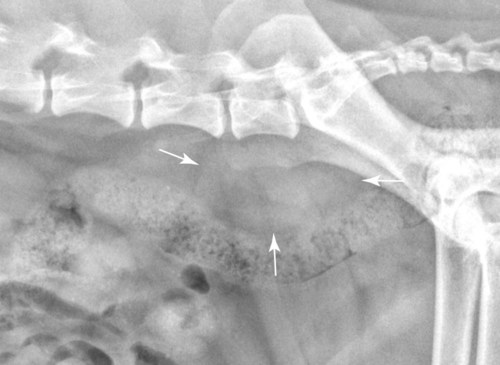
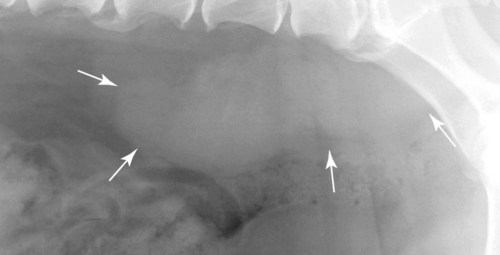
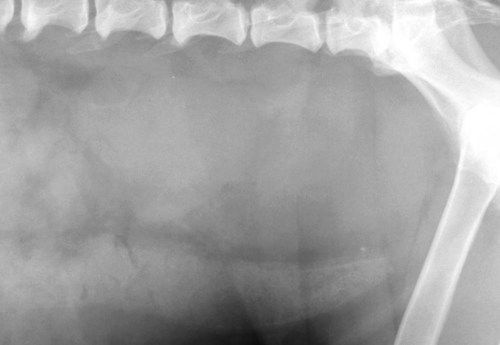
The peritoneum, a thin, serous membrane, is divided into parietal, visceral, and connecting layers, which are all continuous.1 The parietal peritoneum covers the inner surface of the abdominal cavity and is closely adhered to abdominal musculature; it separates extraperitoneal and intraperitoneal spaces. The visceral peritoneum covers the organs of the abdominal cavity either in whole or in part. The connecting peritoneum includes mesenteries, omenta, and intraabdominal ligaments. The peritoneal space, between the parietal and visceral peritoneal layers, normally contains only a small amount of fluid for lubrication. The space between the dorsal margin of the parietal peritoneum and the abdominal wall is the retroperitoneal space. The retroperitoneal space is outside the peritoneal cavity and contains adrenal glands, kidneys, ureters, major blood vessels, and lymph nodes. The retroperitoneal space communicates cranially with the mediastinum through the aortic hiatus and caudally with the pelvic canal.2 Fat is typically present throughout the abdomen, primarily in the falciform ligament, the greater omentum, the mesentery, and the retroperitoneal space. The presence of intraabdominal fat is important for visceral organ visualization in radiographs because fat provides an interposed opacity between viscera (Fig. 36-1). Increased fluid within the peritoneal cavity causes a loss of the differential opacity interface between soft tissue and fat and therefore a loss of contrast between organs. Phrases commonly used to describe this loss of contrast are listed in Box 36-1. Causes for loss of intraabdominal contrast include lack of fat, peritoneal effusion, peritonitis, and peritoneal neoplasia. A wet hair coat, or hair coated with ultrasound gel, may create the appearance of altered peritoneal space opacity. The amount and character of intraabdominal fat depends on the age of the animal and the body habitus. In emaciated patients, the abdomen is often tucked up, which can be visualized on radiographs (Fig. 36-1, C); however, the possibility of coexisting peritoneal fluid or peritonitis cannot be excluded. Normal dogs and cats younger than a few months of age lack sufficient fat to provide intraabdominal contrast; thus the abdomen appears to be of relatively uniform soft tissue opacity (Fig. 36-1, D). Another factor is that young patients have a relatively higher proportion of brown (multilocular) fat than adults. Brown fat has an opacity closer to that of soft tissue because of its higher water content. As young animals mature, the water content of the brown fat decreases.3 As brown fat is replaced by white fat, the contrast between intraabdominal soft tissues increases. Fluid between abdominal viscera, causes border effacement of viscera and a loss of intraabdominal contrast and also provides added overall opacity. Classification of abdominal effusion is broad and includes transudates, exudates, blood, urine, bile, and chyle.4 In practice, all abdominal fluids are of soft tissue opacity, comparable to the visceral organs. In many instances, the fluid is limited to the peritoneal space, and contrast between the kidneys and adjacent retroperitoneal fat is preserved. A large volume of abdominal fluid appears as a homogeneous soft tissue opacity uniformly distributed throughout the abdominal cavity (Fig. 36-2). The homogeneous appearance is caused by border effacement of the soft tissue structures in the abdomen by the fluid. A large volume of fluid will also cause abdominal distention with outward protrusion of the contour of the abdominal wall. The abdomen may also be somewhat pendulous in normal immature patients. A large volume of fluid may also displace the diaphragm cranially. If relatively mobile segments of bowel contain gas, they often float to the highest or uppermost area within the abdominal cavity. These segments will be located in the central portion of the abdomen on a lateral radiograph made with a vertical x-ray beam. Gas-filled bowel loops in a noncentral location in patients with a large amount of intraperitoneal fluid suggest the presence of an abdominal mass or adhesions that prevent free movement of intestinal segments. This finding should be interpreted with caution, however, because the presence of a large amount of peritoneal fat may also cause asymmetric bowel location, especially in cats. The presence or absence of coexistent peritonitis cannot be ascertained radiographically in patients with a large amount of intraperitoneal fluid. Smaller amounts of abdominal fluid or peritonitis may produce a mottled, hazy, or irregular loss of contrast on survey radiographs (Fig. 36-3). Individual viscera may be visualized, but the margins of soft tissue structures are indistinct or blurred. With small amounts of fluid, this appearance may be the result of interdigitation of fluid with folds in the greater omentum and small bowel but without total border effacement.5 Peritonitis may produce a similar effect. Smaller amounts of effusion may be caused by early fluid accumulation of a generalized process or by more localized disease. Localized disease may lead to abnormal serosal margin detail in the area of the disease with normal serosal margins visible elsewhere in the peritoneal space. Manipulation of viscera during laparotomy produces changes that may appear similar to peritonitis, and these changes may be modified by the amount of tissue trauma.5 Solutions containing water, electrolytes, and relatively low molecular weight components are absorbed by the peritoneal membrane within 24 hours.6 Proteinaceous fluids such as serum, blood, and lymph are absorbed more slowly and may be present for 1 to 2 weeks. These changes can be visualized after laparotomy, and they should not be mistaken for more significant complications. Static or increasing fluid accumulation during this period is abnormal. If complications are suspected, cytologic evaluation of the fluid will be necessary to differentiate between normal postsurgical fluid and fluid caused by septic peritonitis or other conditions. One method of assessing the intraperitoneal space for fluid accumulation or peritonitis is to compare the contrast of the intraperitoneal space with that of the retroperitoneal space. Normally, detail and contrast in the intraperitoneal and retroperitoneal spaces should be identical. However, because many diseases resulting in intraperitoneal fluid accumulation do not affect the retroperitoneal space, retroperitoneal detail is often preserved when intraperitoneal fluid has altered the serosal margin of bowel and other intraperitoneal organs (Fig. 36-4). It is important to remember, however, that a large volume of intraperitoneal fluid can obscure the retroperitoneal space because of superimposition. Loss of contrast and detail can also occur specifically in the retroperitoneal space, and is an indication of fluid accumulation or, less commonly, inflammation (Fig. 36-5). Fluid in the retroperitoneal space can lead to alternating fat and soft tissue opacities, or streaking, as the fluid dissects between fascial planes. The most common causes of isolated retroperitoneal fluid are hemorrhage, as with rodenticide toxicity and trauma, and urine leakage. Retroperitoneal inflammation with increased fluid can also result from a migrating grass awn, a penetrating wound, ligatures from ovariohysterectomy, and perforation of the urethra during catheterization.7,8 An ill-defined nodular or granular pattern to the abdomen (Fig. 36-6) may be caused by seeding of the peritoneum with multiple, metastatic neoplastic foci, or it may result from proteolytic enzymes escaping from an inflamed pancreas, causing saponification of omental and mesenteric fat. Examples of tumors associated with such spread include hemangiosarcoma of the spleen and carcinoma of various abdominal organs. The term carcinomatosis may be used to describe any cancer disseminated throughout the abdomen; it may be limited to carcinomas with this distribution, or it can be used as a general term to describe loss of serosal detail with nodularity, as with fat saponification secondary to pancreatitis.9 See Box 36-2 for causes of decreased serosal surface visualization. The two most common causes of free intraperitoneal gas are penetration of the abdominal wall, either by surgery or by penetrating wounds, and perforation of the bowel. However, not all bowel perforations lead to free abdominal gas.10 Laparotomy is the most common cause of free abdominal gas, and the history is usually known in this instance. After laparotomy, a moderate amount of gas may persist for days to weeks.11 Penetrating abdominal wounds are usually diagnosed by physical findings. In patients with a penetrating wound, differentiating whether free abdominal gas is caused solely by penetration of the abdomen or is the result of concurrent organ rupture is impossible to tell from radiographs. If subcutaneous emphysema is superimposed over the abdominal cavity, such as caused by trauma, it may be difficult to discern whether or not there is concurrent intraperitoneal gas. A small volume of free abdominal gas can be difficult to recognize on conventional radiographs made with a vertically directed x-ray beam because the gas bubbles are small and irregular in shape and may be misinterpreted as bowel gas unless they reside in a region where bowel is not found normally (Fig. 36-7).5 Larger gas volumes may coalesce into a larger bubble. This larger bubble may still be difficult to recognize on a radiograph made with a vertical x-ray beam because it is superimposed over other viscera. In addition, this larger bubble may simulate a gas-containing organ, such as the stomach. Free abdominal gas usually floats to the highest point within the abdomen. In lateral recumbency, this is usually under the caudal aspect of the ribs or in the midabdomen. The concurrent presence of abdominal effusion may make recognition of the gas bubble easier because the fluid provides a more uniform, homogeneous soft tissue background opacity (Fig. 36-8, A). Occasionally, the amount of gas will be large enough to outline serosal surfaces of viscera, such as bowel loops, the stomach, and the diaphragm (Fig. 36-8, B). Because free gas rises to the highest point within the abdomen, it may be isolated visually from superimposed structures by a horizontally directed x-ray beam. With a small volume of gas, putting the patient in position for 10 minutes before radiographic exposure may be helpful to allow most of the gas to migrate and coalesce at the uppermost portion of the abdomen. The most sensitive projection for detecting a small volume of peritoneal gas is a lateral view, made with a horizontally directed x-ray beam with the patient in dorsal recumbency and with the cranial portion of the abdomen elevated slightly so small amounts of gas accumulate between the liver, diaphragm, and ventral aspect of the abdominal wall (Fig. 36-9).12 Another projection used for documenting free gas is a ventrodorsal view obtained with the patient in left recumbency with the use of a horizontally directed x-ray beam. Gas will usually collect under the highest portion of the right abdominal wall (Fig. 36-7, B), which is usually under the caudal aspect of the ribs. Raising or lowering either end of the animal shifts the point of gas accumulation. Exposure factors should be lowered to underexpose the abdomen, making the gas more conspicuous. A horizontal-beam view with the animal in right recumbency is not recommended because the gas bubble rises to the left side and gas within the fundus of the stomach may be misinterpreted as free gas. Horizontal-beam radiography is a useful tool for diagnosing free peritoneal gas quickly, cheaply, and with confidence; however, making radiographs with a horizontally directed x-ray beam is technically challenging with many newer digital radiography systems because the imaging plate is large, fragile, and not intended to be moved. However, with patience and care, most imaging plates can be positioned carefully and used for horizontal-beam radiography. Caution should be exercised when moving the imaging plate because it is often the most expensive part of the entire system. One advantage of computed radiography systems compared to direct digital radiography systems is the increased ease of producing radiographs with a horizontal beam. The ease of performing horizontal-beam radiography should be one factor considered when purchasing a new digital radiography system. See Chapter 2 for more information on digital radiography systems. Gas may also accumulate in the retroperitoneal space.8 Retroperitoneal gas is most often the result of extension of pneumomediastinum (see Chapter 30). Retroperitoneal gas is confined to the retroperitoneal space in the dorsal abdomen and is best seen on a lateral radiograph (Fig. 36-10). Increased mineral opacity not associated with the gastrointestinal tract is relatively uncommon. Focal calcified bodies, usually with a more opaque periphery, may be found in the peritoneal space (Fig. 36-11; see Fig. 36-4). These are thought to be the result of dystrophic calcification of necrotic mesenteric fat and are not considered clinically significant.13,14 Although not common, they are seen more often in cats than dogs. These have been referred to as Bates bodies.14 Metastatic calcification of the abdominal vasculature is rare (Fig. 36-12) and is associated with abnormal calcium metabolism, primarily in animals with chronic uremia,13,15 or hypothyroidism. A mineralized fetus may be seen in the peritoneal space with ectopic pregnancy. Clinical signs may be caused by necrosis or mechanical interference, or the condition may be an incidental finding.16 Mineralization may be visualized occasionally in the soft tissues surrounding the abdomen. For example, calcinosis cutis associated with Cushing’s syndrome may produce nodular or linear calcification of soft tissues, most often dorsally and in the ventral abdominal wall.17 Gas from a variety of causes may be seen in the soft tissues surrounding the abdomen. Abrasions with lacerations often produce a mottled, irregular gas pattern. Tubular or round gas pockets may be contained within herniated bowel loops (Fig. 36-13). Ultrasound is extremely useful for evaluating the peritoneal space, especially when increased fluid is suspected. Small fluid volumes can be detected readily and ultrasound guidance can be used to collect samples. Fluid can also be characterized by its echogenicity. Fluid with low cellular content, such as urine or a transudate, is anechoic (Fig. 36-14); fluid with moderate to high cellular content, such as exudate, blood, or chyle, is more echoic (Fig. 36-15). Peritoneal masses can be solid or cavitary, and samples can be obtained for cytologic evaluation. Differentials are similar to those for any mass (e.g., cyst, hematoma, abscess, neoplasia, granuloma). Although uncommon, peritoneal metastases can be detected and appear as fingerlike projections of hypoechoic material scattered throughout the mesentery (Fig. 36-16). In the northwestern portion of the United States and Canada, as well as portions of Europe, peritoneal infection by Mesocestoides species tapeworms may manifest as varying-sized, cavitary, septated structures with echogenic particles within the fluid.18,19 Small volumes of free peritoneal gas can appear as focal artifacts adjacent to the nondependent portion of the peritoneum (Fig. 36-17); they may not interfere with sonographic evaluation, but large volumes of gas hinder complete evaluation of the peritoneal space. Free peritoneal gas can be found sonographically in patients in which it is not diagnosed radiographically, and the specific location and cause of the pneumoperitoneum can be identified more frequently sonographically than in radiographs. The findings of hyperechoic fat, free peritoneal fluid, and a dilated fluid-filled stomach or intestine were considered indirect evidence of gastrointestinal perforation.20 As would be expected, pneumoperitoneum in conjunction with gastric dilatation or volvulus is predictive for gastric necrosis. Prior percutaneous trocharization can confound the situation by leading to pneumoperitoneum and/or pneumatosis mimicking gastric necrosis.21 Abdominal lymph nodes are divided into two groups: parietal and visceral. Parietal lymph nodes lie in the retroperitoneal space and receive afferent lymphatics from the spine, adrenal glands, kidneys, caudodorsal abdomen, pelvis, and pelvic limbs. Efferent vessels from the parietal lymph nodes drain into the lumbar trunk, which in turn empties into the cisterna chyli. The more cranially located of the parietal lymph nodes may bypass the lumbar trunk and drain directly into the cisterna chyli. Many of the parietal lymph nodes are developed inconsistently and may be absent. However, the medial iliac lymph nodes, the largest lymph nodes of the sublumbar group, are constant. The medial iliac lymph nodes, previously known as the external iliac lymph nodes,1 are located ventral to the vertebra and between the deep circumflex iliac and external iliac arteries. Although medial iliac lymph nodes are reported to lie ventral to L5 and L6,1 these lymph nodes often are located ventral to L6 and L7.5 One lymph node is usually present on each side, but occasionally two lymph nodes are on one or both sides. The medial iliac lymph nodes receive afferent lymphatics from the urogenital tract as well as from other structures in the caudal abdomen, pelvis, and pelvic limbs. Abdominal lymph nodes are seen radiographically only if they are enlarged or mineralized. Abundant retroperitoneal fat helps provide contrast between enlarged lymph nodes and surrounding soft tissue structures. Of the parietal lymph nodes, the medial iliac nodes are usually the only lymph nodes that enlarge to the degree that they are seen radiographically. Care should be taken not to confuse the deep circumflex arteries and veins that are projected end-on in lateral radiographs as enlarged medial iliac lymph nodes (Fig. 36-18). Enlarged medial iliac lymph nodes appear as a soft tissue mass in the retroperitoneal space ventral to L6 and L7 (Fig. 36-19). If node enlargement is severe, the lymph nodes are more conspicuous and extend more cranially (Fig. 36-20). Enlarged lymph nodes frequently displace the descending colon and rectum ventrally (Fig. 36-21). However, a ventral course of the colon is not an indication of medial iliac lymph node enlargement unless a soft tissue mass is present in the expected location of the lymph nodes because the colon may be positioned more ventral than usual without being displaced by a mass. The most common cause of medial iliac lymphadenopathy is neoplasia. Neoplastic lymph node involvement may be primary (e.g., lymphosarcoma) or metastatic (e.g., from caudal abdominal or pelvic neoplasms).22 Inflammatory disease may also cause enlargement of the medial iliac lymph nodes, but this is unusual. Ultrasound is more sensitive than radiography for imaging lymph nodes. Because the medial iliac and jejunal lymph nodes are the largest and most consistent in the abdominal cavity, they can be found more often than other lymph nodes when normal.23 Normal lymph nodes have an echogenicity similar to surrounding mesentery and adjacent musculature,23 but knowledge of their location and careful identification of elongated structures of uniform echotexture and thin echogenic capsules can permit detection (Fig. 36-22). High-frequency transducers are necessary to obtain resolution adequate for imaging normal lymph nodes. Lymph nodes are generally identified more easily in young or thin animals.23 When abnormal, lymph nodes tend to enlarge and become more round and hypoechoic (Fig. 36-23, see page 668).23–25 Neoplastic lymph nodes tend to have a short-to-long axis ratio of more than 1:2, narrow or absent hilus, hypoechogenicity, sharp borders, a resistive index (RI) greater than 0.65, a pulsatility index (PI) greater than 1.45, and often distal acoustic enhancement. These findings tend to not be present in reactive lymph nodes.25
The Peritoneal Space
Peritoneal Space
Loss of Contrast Caused by Increased Fluid Opacity
Increased Contrast Caused by Increased Gas Opacity
Intraabdominal Mineral Opacity
Abdominal Wall Abnormalities
Sonography of the Peritoneal Space
Abdominal Lymph Nodes
Abnormalities of Lymph Nodes
Sonography of Parietal and Visceral Lymph Nodes
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


The Peritoneal Space