Chapter 2 The operating room
Introduction
chapter 1.2, chapter 1.3, chapter 1.4, chapter 1.5 and Box 1.1 list the different instruments for the varying ophthalmic surgeries. For more specialized surgeries, such as retinal detachment surgeries (see Table 1.5), instruments are often wrapped and sterilized separately.
Magnification
Head-mounted magnifiers
The exact requirements for magnification vary and are often influenced by the surgical patient load and the different types of ophthalmic surgical procedure performed. The simplest and least expensive magnification device is the binocular magnifier loupe worn on the head (Fig. 2.1). These head loupes can be used over prescription glasses. The head loupe is available in a number of different magnifications. Generally the lower magnifications are the most versatile because at the higher magnifications the focal length of the loupe is reduced, thereby limiting the working distance between the surgeon and the operating field. For instance, the head loupe with the 1.5× magnification has a focal length of 51 cm; the 1.75× magnification has a focal length of 35.5 cm; the 2× magnification is in focus at 25.5 cm; and the 2.5× magnification has a focal length of 20.5 cm.
Individual telescope magnifiers are another low-cost alternative, and are generally recommended. They can be added to head loupes or attached directly to spectacles. These units permit accommodation for different interpupillary distances, allow use of prescription glasses, and can be elevated when not in use (Fig. 2.2). Although these units are lightweight, use for several hours when attached to spectacles can be very tiring.
Operating microscopes
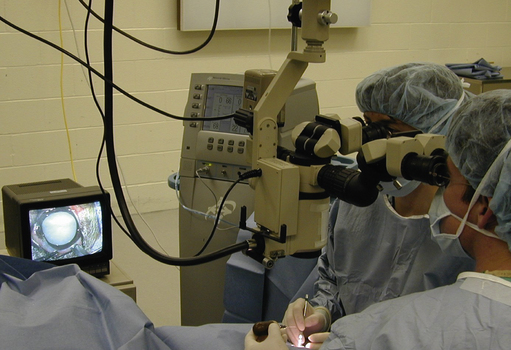
Operating microscopes can be portable and attached to either a table or floor base with casters. Table units are the least expensive, usually provide observation for only the surgeon, and changes in focus require manual adjustments (Fig. 2.3). Stationary ceiling-mounted operating microscopes are used infrequently in veterinary ophthalmology because of the need for portability between operating rooms. Most veterinarians use the floor-based operating microscopes which are very stable but mobile (Fig. 2.4).
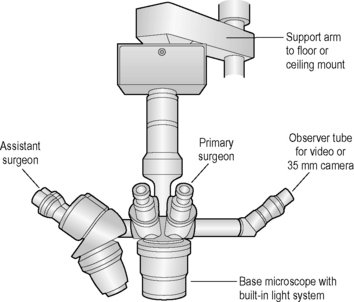
The operating microscope has several standard parts (Fig. 2.5). The base and mount are usually quite heavy and vary depending on whether the unit is table, floor or ceiling mounted. Various supporting arms permit adjustment of the operating microscope’s main body over the patient’s eye and angulation of the scope to the surgical field. With a large footplate that contains several switches, the surgeon can raise and lower the main body of the operating microscope to permit motorized coarse and fine focus of the surgical field. The scope’s main body consists of the focus and zoom systems, and a beam splitter that permits observation of the surgical field by the surgeon and assistant surgeon, and often a video recorder or 35 mm or digital camera. The fine focus of the surgical field and zoom or magnifying system are also controlled by a foot pedal. This allows slight adjustments on magnification and/or focus without interrupting surgery.
Chairs for microsurgery
All corneal and intraocular surgical procedures in small animals are performed with the surgeon and assistant surgeon seated on adjustable stools with casters (Fig. 2.6). The height of the stools should be adjustable to accommodate different surgeons as well as the height of the operating table and the patient. The recommended operating room chairs can be adjusted with a hydraulic activated foot pedal, permitting changes during surgery without interrupting surgery. In some stools the back rest can be rotated 180° and moved to the front of the surgeon to provide arm rests during microsurgery. These arm rests can be covered with sterile stockinet. The operating chairs for the surgeon and assistant surgeon should be comfortable and help to avoid fatigue that can adversely impact surgery.
Patient preparation
Head positioning
The majority of corneal and intraocular surgical procedures are performed with the small animal patient in dorsal recumbency. If head-mounted magnifiers are worn, the dog may be positioned in lateral recumbency. Ropes and adhesive tape are used to position the animal’s legs. Although the duration of most ophthalmic surgical procedures is less than 1 h, a circulating water heating pad between the patient and the surgery table reduces the possibility of hypothermia. It is important to place the animal’s head in a secure position to prevent any positional changes during surgery. Towels, water bottles, or several small low-cost sandbags can be used to maintain the head in the desired position. An alternative superior scheme uses a vacuum bead-filled U-shaped pack for stabilizing the head (Fig. 2.7). The patient’s head is positioned on the vacuum pack, and the pack is manipulated to provide the selected head position. Once the proper position is achieved, vacuum is applied temporarily to the pack. Once the air is removed, the pack becomes very rigid, holding the head in a fixed position. Release of the vacuum postoperatively causes the pack to return to a soft and moldable structure.
Basic operative approach
Retrobulbar injections in dogs
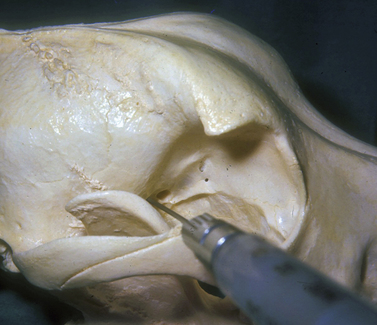
The injection is performed with the dog under general anesthesia with the objective of forcing the globe further rostrad or forward in the orbit, or to turn the globe and improve exposure of a selected area of the cornea and/or anterior segment. The amount of sterile saline injected is ascertained as the injection is performed and the response of the globe to the space-occupying solution. The hypodermic needle may be inserted caudal to the junction of the lateral orbital ligament and dorsal aspects of the zygomatic arch (Fig. 2.8). The needle is directed towards the retrobulbar space in a ventromedial direction toward the opposite mandibular joint. The solution may be injected in the lateral aspects of the extraocular muscle cone, or immediately caudal to the globe and within the retrobulbar muscle mass. Injections external to the retrobulbar muscle cone will rotate the globe laterally; injections immediately behind the globe will push the globe forward. The volume injected should be limited to produce the desired outcome but not result in undue pressure and distortion of the globe.
Another injection site is ventral to the anterior zygomatic arch and rostrad to the vertical portion of the ramus of the mandible (see Chapter 3). The hypodermic needle, after passing the ramus of the mandible, is directed toward the orbital fissure. Injections external to the retrobulbar muscle cone in the orbital floor and the medial orbit wall are possible with this method, and can be used to shift the globe dorsally.
Retrobulbar injections in horses
In the Berge method, an 8–10 cm, 18 g needle is inserted caudal to the supraorbital process of the frontal bone near the supraorbital foramen. The long needle is directed ventromedial (about 40° from the vertical) and slightly caudal toward the area of the orbital fissure where 15–20 mL of local anesthetic is injected (see Chapter 3).
In Lichenstern’s method, an 8–10 cm, 18 g needle is inserted 1.5 cm caudal to the middle of the supraorbital process. The needle is directed toward the opposite last upper premolar tooth. The taut extraocular muscles’ fascial cone may be felt as the needle penetrates it. Approximately 20 mL of local anesthetic is injected near the orbital fissure (see Chapter 3).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree