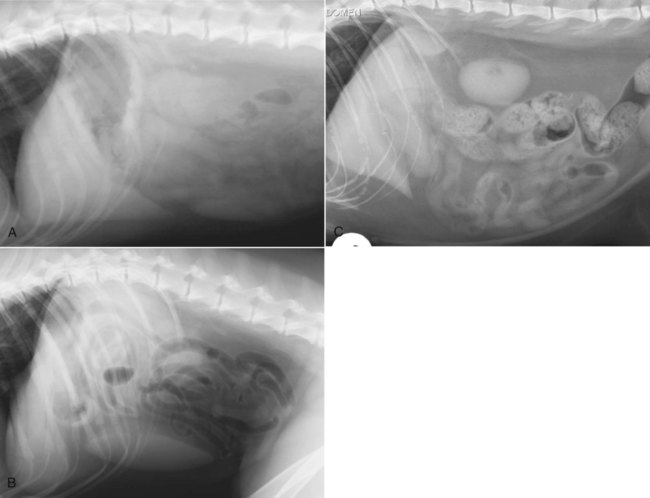
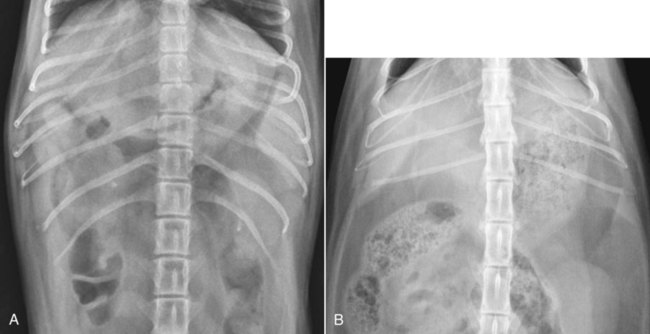
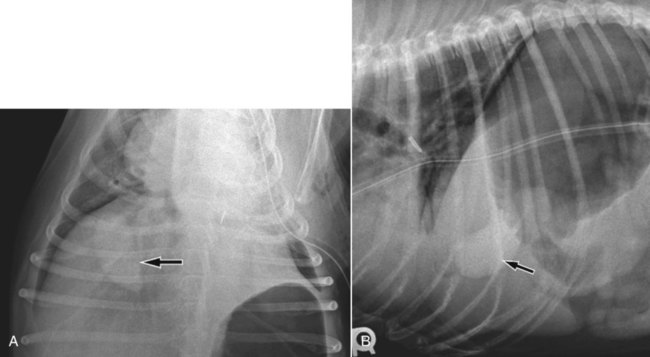
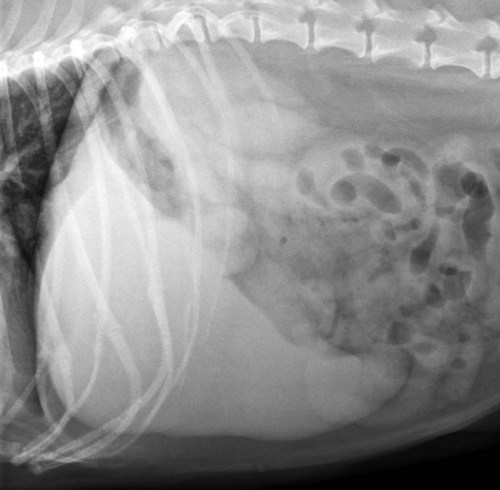
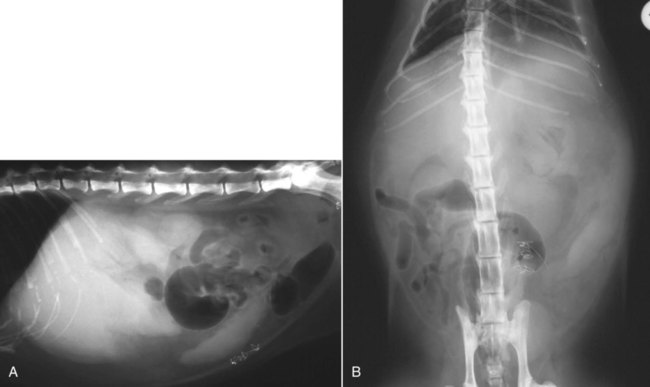
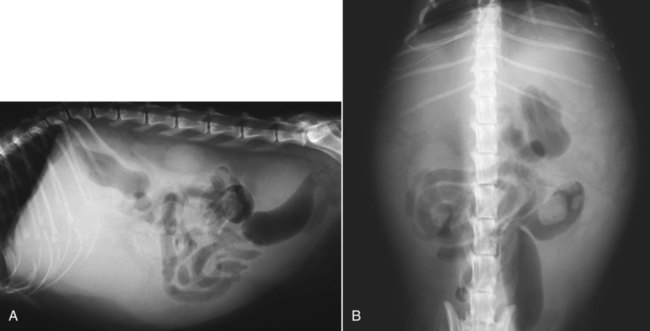
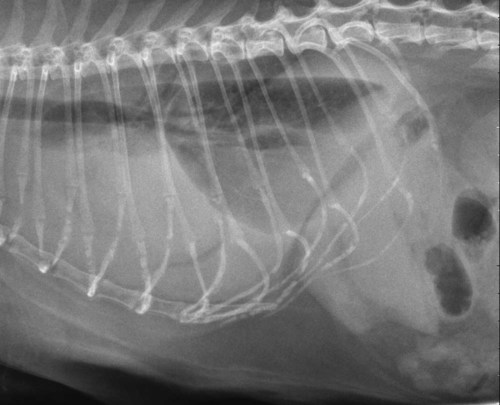
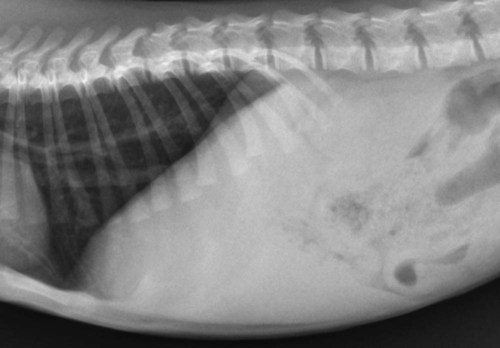
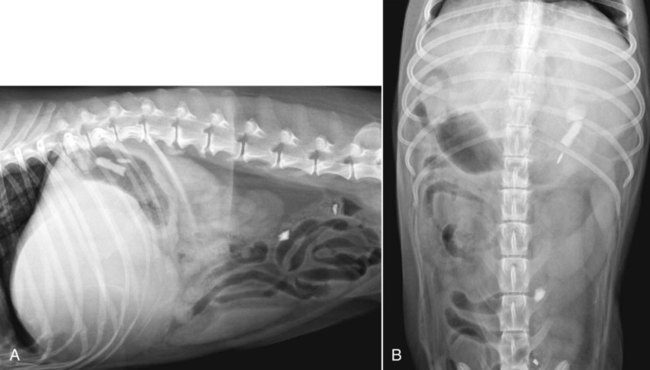
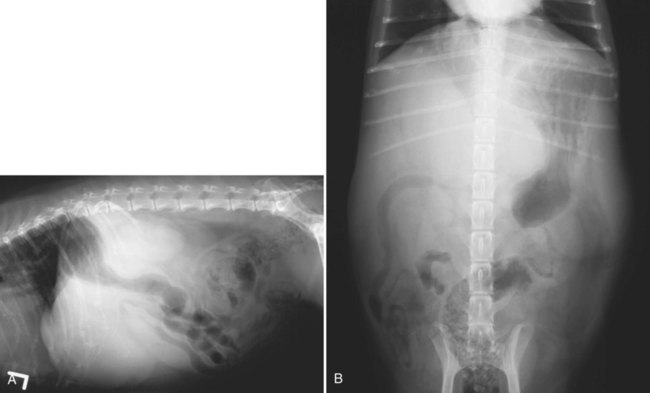
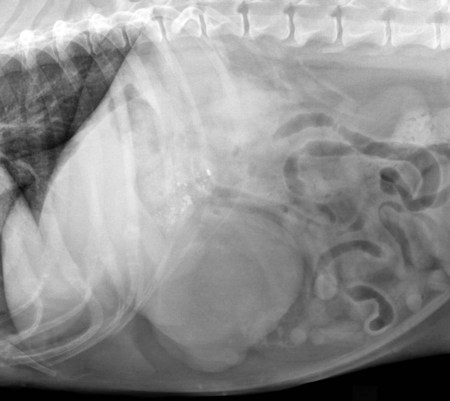
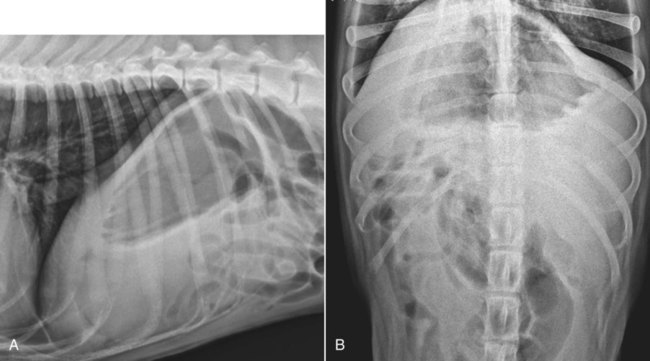
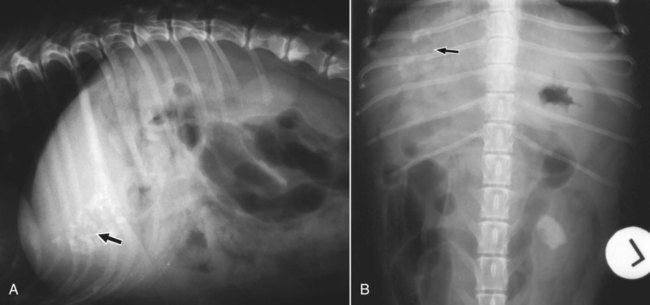
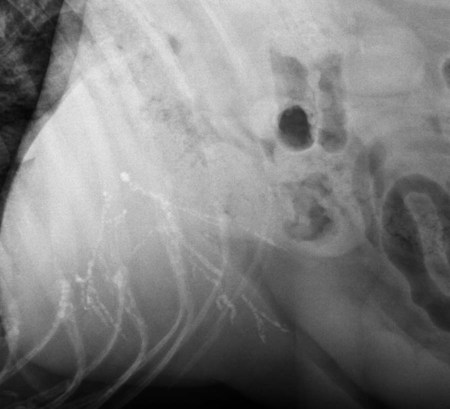
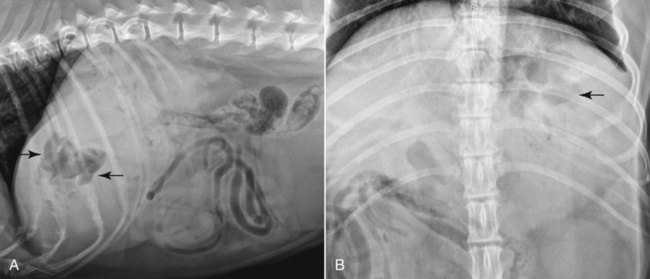
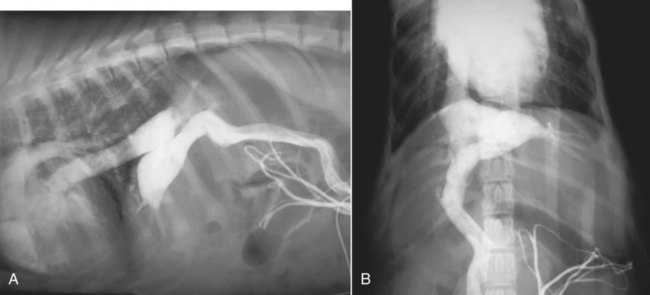
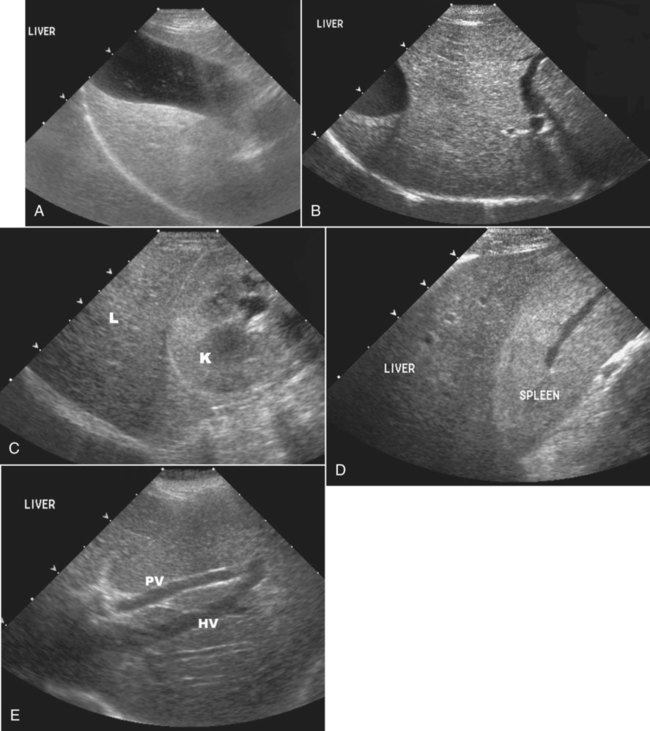
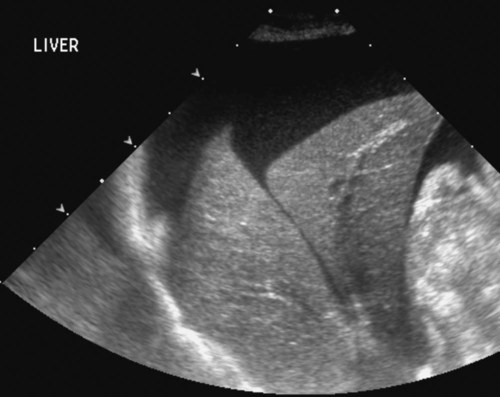
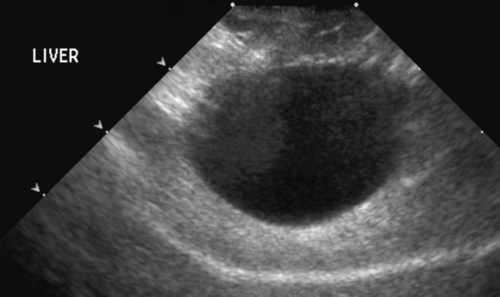
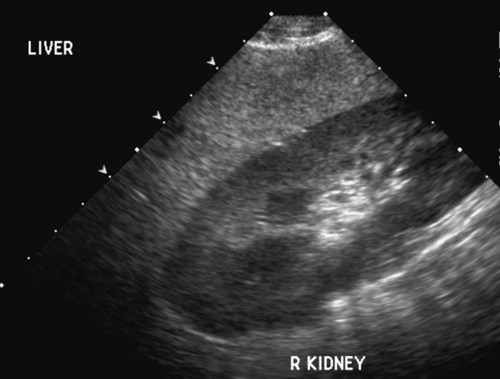
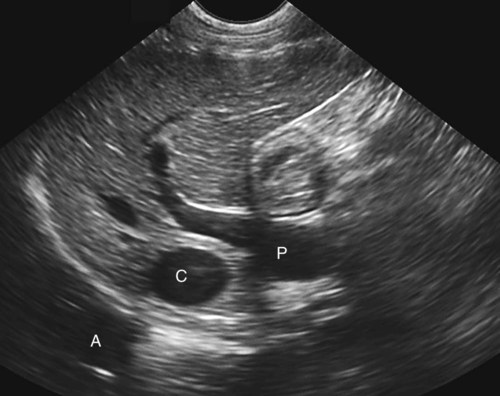
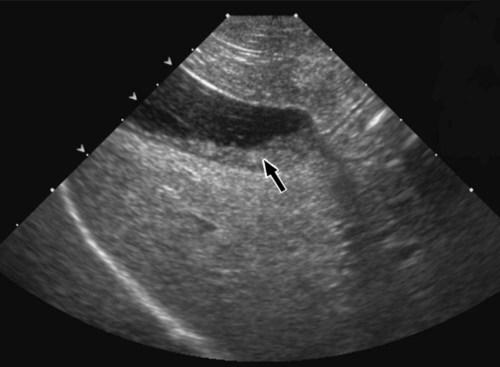
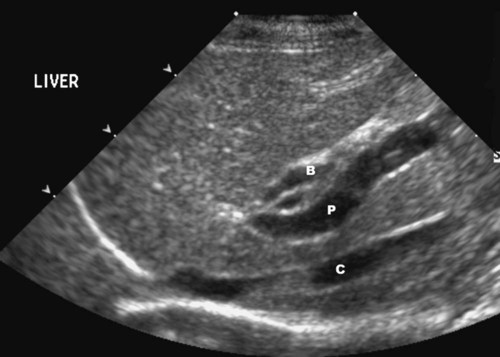
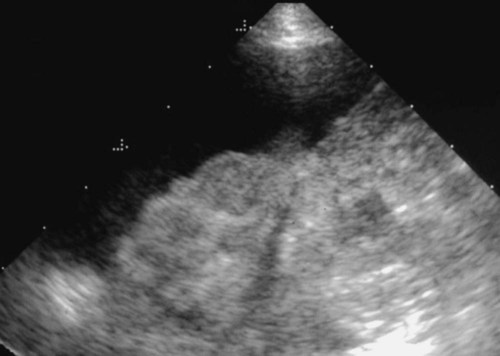
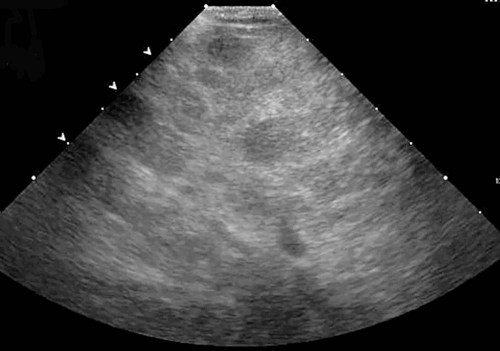
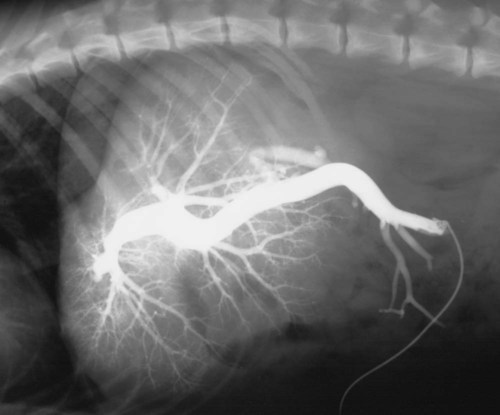
The liver is the largest solid organ in the abdomen. Changes in hepatic size, shape, location, and opacity are used to assess the liver for possible abnormality.1–7 The liver is located in the cranial aspect of the abdomen between the diaphragm, which delineates its cranial border, and the stomach, right kidney, and cranial portion of the duodenum, which define the caudal extent. The liver is nearly entirely within the costal arch, with the caudal ventral border, composed of the left lateral liver lobe in the dog, extending just slightly beyond the costal arch (Fig. 37-1). In dogs with a deep thoracic cavity, the liver lies more completely within the costal arch, whereas greater caudal hepatic extension is present in dogs with shallow, wide thoracic conformation. Abundant falciform fat, especially in cats, can result in dorsal displacement of the ventral aspect of the liver on lateral views. On ventrodorsal views, the liver is distributed fairly symmetrically in dogs, but a larger portion is often right sided in cats (Fig. 37-2). The gallbladder is located just to the right of midline, in the cranioventral portion of the liver, but is not visible normally because of silhouetting with the soft tissue of the liver (Fig. 37-3). In some cats, however, the gallbladder can be seen on lateral abdominal radiographs as a curved structure protruding from the ventral liver margin.8 Hepatic enlargement can be detected radiographically, although mild size changes cannot be assessed accurately. The classic radiographic signs of generalized hepatomegaly are rounding or blunting of the caudoventral liver margins, along with extension beyond the costal arch, and caudal, and perhaps medial, displacement of the gastric axis (Figs. 37-4 to 37-6).9–11 Several nonpathologic conditions can result in extension of hepatic margins beyond the costal arch, including overexpansion of the thorax or deep inspiration (Fig. 37-7). Older dogs and cats can have stretching or elongation of the triangular ligaments attaching the liver to the diaphragm, resulting in sagging and caudal extension of the liver. The same phenomenon can occur in obese dogs with a pendulous abdomen. In the obese dog, the liver does not extend as far dorsally. Some brachycephalic and chondrodystrophic dogs have caudal extension of the liver as a result of it being aligned more horizontally compared with deep-chested breeds. In addition, neonatal and young dogs and cats have a larger liver size compared with body size, creating the appearance of hepatomegaly without a true hepatic abnormality (Fig. 37-8).1,12 Because of the numerous normal variations that can cause hepatic lobe extension beyond the costal arch, rounding or blunting of these lobes should also be present before hepatomegaly is concluded. With generalized hepatomegaly, caudal displacement of the stomach, right kidney, transverse colon, and cranial duodenal flexure may occur, along with dorsal elevation of the pylorus. On ventrodorsal views, an increased opacity may be present in the right cranial abdominal quadrant along with displacement of the body and pyloric portion of the stomach caudally, and to the left. Both lateral and ventrodorsal abdominal views should be examined to evaluate liver size because hepatomegaly is sometimes obvious only on one view. The position of the stomach is important in determining hepatomegaly, but the stomach may be poorly visualized if empty of gas or food. Administration of a small amount of barium (1 mL/kg) to define the gastric position can help evaluate hepatic size.7 Visualization of focal hepatomegaly depends on the degree of enlargement and the lobe affected. Focal hepatic masses usually result in distortion of the hepatic outline and are continuous with the liver in at least one projection.9–11 Right-sided hepatic masses displace the stomach and duodenum to the left and dorsally and the small bowel caudally (Figs. 37-9 and Fig. 37-10). The right kidney and distal extremity of the spleen may also be displaced caudally by a right-sided hepatic mass. Left hepatic masses result in displacement of the stomach and spleen dorsally and to the right. With few exceptions, masses located cranial to the ventral aspect of the stomach are hepatic in origin.9 Although hepatic masses classically result in caudal displacement of the stomach, a focal mass can extend caudal to the stomach (Fig. 37-11).10,11 Differentiation of a caudally located hepatic mass from a splenic mass based on radiographs alone is difficult in these instances. Differentials for focal hepatic masses include primary and metastatic neoplasia, abscess, granuloma, and hepatic cyst. As with subtle hepatomegaly, slight decreases in hepatic size are not identified accurately radiographically. Marked microhepatia results in cranial displacement of the stomach and decreased distance between the diaphragm and gastric lumen (Fig. 37-12). Congenital portosystemic shunts and hepatic cirrhosis are the two most common causes of microhepatia. Diaphragmatic hernia with displacement of the liver cranial to the diaphragm can give the appearance of a small liver, but there will be intrathoracic abnormalities in this instance. The normal liver is of soft tissue opacity. Mineral opacities can occur in the hepatic parenchyma or biliary system.13 Choleliths should be considered when focal mineral opacities are visible in the area of the gallbladder (Fig. 37-13). Linear trails of mineralized opacities extending peripherally are indicative of choledocholiths.14 Biliary calculi are uncommon in dogs and cats but are visible radiographically if they contain sufficient calcium (Fig. 37-14).15–22 These are usually incidental findings, but choledocholiths can cause biliary obstruction. Mineralization of the gallbladder wall has been associated with gallbladder carcinoma as well as cholecystitis or cystic mucinous hyperplasia.13,23 Hepatic parenchymal mineralization may be localized or diffuse and have a variety of patterns.13 Dystrophic calcification of hepatic granulomas, abscesses, hematomas, neoplastic masses, or areas of hepatic necrosis have been documented (see Fig. 37-10). Mineralization of the biliary tree is seen occasionally in dogs with bile duct carcinoma.24 Echinococcosis infection can result in large hepatic soft tissue masses with mineralization of varying patterns and should be considered in endemic areas.25 Radiolucent areas within the liver are indicative of intrahepatic gas, either in the biliary system, portal venous system, or hepatic parenchyma. Gas within portal vessels may occur as a result of severe necrotizing gastritis or enteritis, often associated with gastric dilation and volvulus complex. Gastrointestinal ulceration, distention, trauma, or interventional procedures may allow gas to ascend into the portovenous circulation.1,26,26 A linear, branching radiolucent appearance, similar to air bronchograms, may be visible. Gas in or around the gallbladder occurs with emphysematous cholecystitis and occurs in both diabetic and nondiabetic dogs.28,29 Gas is seen initially in the gallbladder wall, followed by more complete filling of the lumen. The gas eventually extends to the pericholecystic tissues. Gas bubbles conforming to the shape of the distended gallbladder can be seen within 24 to 48 hours of onset of disease. Obstruction of the cystic duct may be a common predisposing factor for emphysematous cholecystitis. Gas lucencies within the biliary system can also be seen after surgery of the duodenum or biliary system.1 Incidental reflux of gas into the bile duct from the duodenum is occasionally seen in cats. This may be because of incompetence of the sphincter of Oddi.4 Hepatic abscesses caused by gas-forming organisms may result in gas opacities within the hepatic parenchyma.30–33 These abscesses appear as irregularly stippled or mottled gas patterns, usually in a localized area (Fig. 37-15). Hepatomegaly or hepatic mass is typically present with hepatic abscess with or without gas formation. Portosystemic shunts are congenital or acquired anomalies of the portal vasculature in which blood bypasses the liver and enters the systemic circulation directly.34 Various imaging techniques have been used to identify and characterize the anomalous vessels, including cranial mesenteric portography, percutaneous splenoportography, and operative mesenteric portography.34–37 Portography, where the portal system is opacified with iodinated contrast medium, provides visualization of the anomalous vessel, any acquired collateral vessels, the direction of portal blood flow, and patency of the portal vein and its branches. Intraoperative mesenteric portography involves intraoperative catheterization of a jejunal vein, or sometimes a splenic vein, to outline the portal system (Figs. 37-16 and 37-17). Ultrasound-guided percutaneous splenic vein catheterization can also be used but this may be challenging, especially in smaller patients.38 Patient position during contrast-medium injection may have an effect on the visualization of the opacified vessels as a result of gravity-dependent alterations in the distribution of portal blood flow. Contrast-medium injection should be performed with the patient in left lateral and dorsal recumbency, followed by a repeat injection in right lateral recumbency if the results of the first two injections are negative or inconclusive.39 The use of postshunt ligation intraoperative mesenteric portography provides confirmation that the shunt has been identified correctly and ligated as well as information on the extent of hepatic portal vasculature.40 With increased availability of high-quality computed tomography scanners, abdominal computed tomography angiography is replacing most invasive portography procedures for assessment of the portal venous system. This technique provides portogram-like images of the normal and abnormal vasculature.41,42 The hepatic parenchyma has medium-level echogenicity with a homogeneous and uniform texture that is somewhat coarser than in the spleen.43–47 The normal echogenicity of the liver is isoechoic to either slightly hyperechoic or slightly hypoechoic to the renal cortex and hypoechoic to the spleen (Fig. 37-18). The cranial pole of the right kidney provides a good reference point for assessing liver echogenicity in the immediately adjacent caudate liver lobe. Hepatic echogenicity is a subjective assessment, and mild changes should be interpreted with caution. The liver margins should be smooth and sharp but are better visualized if adjacent peritoneal fluid is present (Fig. 37-19). The liver is bordered cranially and dorsally by an echogenic line representing the interface between the diaphragm and lung/pleura. A mirror-image artifact is frequently noted deep to the diaphragmatic interface, giving the false impression of liver on both sides of the diaphragm (see Chapter 3 for a detailed explanation of this artifact). The ultrasound assessment of liver size is subjective and based on operator experience.48 A small liver is difficult to evaluate sonographically because of cranial displacement of the stomach, limiting the imaging window (Fig. 37-20). Liver size may appear decreased in dogs with a deep thoracic cavity wherein liver location is more completely within the costal arch. Intercostal ultrasound windows may be needed in these patients. The enlarged liver can be examined relatively easily with ultrasound because it extends well beyond the xiphoid cartilage and covers the right kidney more completely (Fig. 37-21). Liver margins may appear rounded and may extend beyond the left lateral margin of the stomach. Hepatic and portal veins are visualized routinely within hepatic parenchyma. Portal veins are smoothly tapering vessels characterized by bright, echogenic borders.43–46,49 The larger left and smaller right branch originate from the main portal vein near the porta hepatis, although they branch in different imaging planes.49 Hepatic veins are anechoic linear structures extending through the parenchyma. Hepatic vein borders are not echogenic with the exception of their confluence with the caudal vena cava, immediately adjacent to the diaphragm. The right lateral dorsal intercostal window provides an excellent window to the aorta, caudal vena cava, and main portal vein (Fig. 37-22). Normal hepatic arteries are not visualized easily without color Doppler examination. The caudal vena cava can be visualized coursing through the liver in the right lateral abdominal quadrant. The gallbladder is well visualized as an oval, anechoic structure in the right cranioventral portion of the liver. Gallbladder size varies widely, and distention is normal in fasting or anorexic patients. Intraluminal contents are typically anechoic, although gallbladder sludge, which is dependent echogenic material without acoustic shadowing, is seen frequently and is usually an incidental finding (Fig. 37-23).50 The normal gallbladder wall is thin and poorly visualized. In the cat, gallbladder wall thickness should be less than 1 mm or not visualized at all.51 The normal canine gallbladder wall typically measures 2 to 3 mm, but normal ranges have not been established.52,53 A duplicate or septated gallbladder is seen occasionally as a normal variation in cats and is caused by abnormal embryonic development.54 The common bile duct is immediately ventral to the portal vein but is visible more consistently in the cat, where it can usually be followed to the duodenal papilla (Fig. 37-24). Normal common bile duct diameter in the cat is 4 mm or less.55 If visible, the canine bile duct should be 3 mm or less.56 Intrahepatic bile ducts are not visible unless dilated pathologically. Ultrasound is helpful in differentiating between diffuse and focal hepatic disease. Diffuse hepatic disease can result in changes in shape, size, and echogenicity.43–46,57–61 A hyperechoic liver is identified by comparison with the echogenicity of an adjacent organ, such as liver being hyperechoic to renal cortex, or isoechoic or hyperechoic to spleen. Also, there will be loss of visualization of the conspicuous periportal echoes and increased attenuation of sound as it passes through the hyperechoic liver. Vacuolar hepatopathies, including lipidosis and steroid hepatopathy, result commonly in an enlarged, hyperechoic liver (see Fig. 37-21). In cats, liver parenchyma that is hyperechoic to adjacent falciform fat is suggestive of hepatic lipidosis, but this may be normal in some obese cats.59,62 Chronic hepatitis with parenchymal fibrosis can also cause increased echogenicity, although liver size is variable and may be normal, increased, or decreased. Hepatic cirrhosis typically results in a small, irregular, hyperechoic liver (Fig. 37-25). Ascites often accompanies cirrhosis, enhancing visualization of irregular liver margins. Other hepatic diseases that may result in increased parenchymal echogenicity include lymphosarcoma, amyloidosis, and cholangiohepatitis. Mast cell infiltration in the liver results in a variable appearance, from normal to increased echogenicity and size. Hypoechoic nodules are occasionally noted as well.63 A diffuse mottled hepatic parenchyma, characterized by a hyperechoic background with poorly defined hypoechoic nodules, is seen commonly with various combinations of vacuolar hepatopathy, nodular hyperplasia, and chronic inflammation (Fig. 37-26). A decrease in hepatic echogenicity results in increased periportal echoes and abnormal comparison to the renal cortex because the liver becomes hypoechoic to the cortex. Decreased hepatic echogenicity occurs with hepatic congestion, lymphosarcoma, and cholangiohepatitis; a dilated caudal vena cava and hepatic veins will accompany hepatic congestion. Acute suppurative hepatitis can cause hypoechogenicity from inflammation and edema. However, this may be uncommon, even with severe disease.64 Of note, a normal hepatic ultrasound examination does not rule out diffuse hepatic disease because structural changes need to be severe before ultrasound changes are visible. Ultrasound appears to be relatively insensitive in detecting parenchymal changes of hepatic lymphosarcoma.65,66 Subtle changes in hepatic echogenicity should be compared with clinical signs and laboratory data, and a biopsy is necessary for definitive diagnosis.67,68 Prebiopsy coagulation screening is a useful precaution when liver disease is suspected. Focal hepatic disease appears as a nodule or mass that differs in texture and echogenicity from surrounding normal liver parenchyma. They may interrupt the hepatic margin, resulting in change of shape or contour. Relatively small nodules can be detected, especially when using high-frequency transducers. However, although ultrasound is sensitive in detecting hepatic nodules, it is not specific and numerous considerations are possible for focal disease. Cysts, abscesses, primary or metastatic neoplasia, hematomas, granulomas, nodular hyperplasia, and extramedullary hematopoiesis can all produce focal hepatic disease and may be difficult to differentiate on the basis of ultrasound appearance alone.43–46,59 However, ultrasound is extremely useful in differentiating cystic versus solid masses; focal, multifocal, or diffuse distribution of masses; the relation of the mass to adjacent structures, such as large blood vessels or the gallbladder; and assessment of tumor vascular patterns with Doppler imaging techniques.11 Hepatic neoplasia has a variable appearance.43–46,69 Primary hepatic carcinoma may appear hypoechoic, hyperechoic, or of mixed echogenicity (Fig. 37-27). Primary neoplasia may be a solitary large mass confined to a single liver lobe; multifocal, involving several lobes; or multifocal or coalescing nodules in all liver lobes.11,70 Hepatic lymphosarcoma, although sometimes seen as a change in size and echogenicity, can also result in focal nodules, usually hypoechoic.65,66
The Liver and Spleen
Radiology of the Liver
Hepatomegaly
Hepatic Opacity
Special Radiographic Procedures of the Liver
Hepatic Ultrasound
Abnormal Sonographic Appearance of the Liver
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


The Liver and Spleen