Tick families and genera
Hoogstraal (1985)
Horak et al. (2002)
Barker and Murrell (2004)
Nava et al. (2009)
Guglielmone et al. (2010)
Mans (2011)
Nuttalliella
1
1
1
Omitted
1
1
Argasidae
Argas
56
57
57
60
61
60
Antricola
8
Omitted
Omitted
17
17
17
Nothoaspis
1
Omitted
Omitted
1
1
1
Ornithodoros
100
38
37
106
112
45
Otobius
2
3
3
Omitted
2
2
Carios
Omitted
87
88
Omitted
Omitted
Omitted
Alectorobius
Omitted
Omitted
Omitted
Omitted
Omitted
66
Ixodidae
Amblyomma*
102
129
142
129
130
130
Anomalohimalaya
3
3
Omitted
Omitted
3
3
Aponoma
24
Omitted
Omitted
Omitted
Omitted
Omitted
Boophilus*
5
Subgenus
Subgenus
Omitted
Omitted
Omitted
Cosmioma
1
1
1
1
1
1
Dermacentor
30
33
36
33
34
34
Haemaphysalis
155
168
166
164
166
166
Hyalomma
30
30
25
25
27
27
Ixodes
217
243
249
242
243
243
Margaropus
3
3
3
3
3
3
Nosoma
1
1
1
1
2
2
Rhipicentor
2
2
2
2
2
2
Rhipicephalus*
70
80
79
81
82
82
Bothriocroton*
Omitted
5
5
7
7
7
8.2 The Genus Hyalomma: Updated Species Names
Hoogstraal (1956) stated the following: Persons attempting to identify field collected material of Hyalomma should recognize that a certain proportion of specimens in many series will defy final determination of species. These had best be called ‘Hyalomma species’. He also referred to the heterogeneity of individuals within a species as it may have originated from the ‘genetic instability in many specimens’. Since that time until to date, the ‘criteria for identification to species level is in a chaotic state’. Apanaskevich and Horak, in series of publications from 2002 to 2010, attempted to review the taxonomy of the genus using the first author’s expertise in the taxonomic status of ticks belonging to the genus Hyalomma. Moreover, he had access to the largest tick collection in the world, the United State National Tick Collection, Georgia Southern University. The following review is an attempt to update the species names up to the year 2011.
1.
Hyalomma aegyptium Linnaeus, 1758.
This is the type species of the genus Hyalomma, as discussed in Filippova, 1984.
2.
Hyalomma albiparmatum Schulze, 1919.
3.
Hyalomma anatolicum Koch, 1844.
4.
Hyalomma arabica Pegram, Hoogstraal and Wassef, 1982.
5.
Hyalomma asiaticum Schulze and Schlottke, 1930.
6.
Hyalomma brevipunctata Sharif, 1928.
7.
Hyalomma dromedarii Koch, 1844.
8.
Hyalomma excavatum Koch, 1844.
This species was previously classified as a subspecies of H. anatolicum. It was recognized as a subspecies of H. anatolicum by Camicas et al. (1998) and Horak et al. (2002). It is omitted in the valid list by Barker and Murrell (2004). It is re-described and given species status by Apanaskevich and Horak (2005).
9.
Hyalomma franchinii Tonelli-Rondelli, 1932.
10.
Hyalomma glabrum Delpy, 1949.
11.
Hyalomma hussaini Sharif, 1928.
12.
Hyalomma hystricis Dhanda and Raja, 1974.
13.
Hyalomma impeltatum Schulze and Schlottke, 1930.
14.
Hyalomma impressum Koch, 1844.
15.
Hyalomma isaaci Sharif, 1928.
16.
Hyalomma kumari Sharif, 1928.
17.
Hyalomma lusitanicum Koch, 1844 18.
18.
Hyalomma marginatum Koch, 1844.
This species was considered as a subspecies of H. marginatum Koch, 1844. It is now raised to a species level by Apanaskevich and Horak (2008a).
19.
Hyalomma nitidum Schulze, 1919.
Apanaskevich and Horak (2008b) considered H. nitidum a valid species.
20.
Hyalomma punt Hoogstraal, Kaiser and Pedersen, 1969.
21.
Hyalomma rhipicephaloides Neumann, 1901.
22.
Hyalomma rufipes Koch, 1844.
23.
Hyalomma schulzei Olenev, 1931.
24.
Hyalomma scupense Schulze, 1918; Apanaskevich et al. (2010).
This species was considered a subspecies of H. detritum Schulze, 1919. Because this is an economically important tick, Guglielmone et al. (2009) proposed referring to it as H. scupense (= H. detritum) in order to avoid confusion. H. detritum, as a valid tick species was synonymised with H. scupense by Apanaskevich et al. (2010).
25.
Hyalomma somalicum Tonelli-Rondelli, 1935.
This taxon was recently resurrected by Apanaskevich and Horak (2009) for H. erythraeum, Kaiser and Hoogstraal, 1968 (see H. impeltatum).
26.
Hyalomma truncatum Koch, 1844.
27.
Hyalomma turanicum Pomerantzev, 1946. This species was previously classified as a subspecies of H. marginatum. Listed by Horak et al. (2002) and Barker and Murrell (2004) as subspecies and was omitted in the list by Camicas et al. (1998). It is confirmed as a valid species by Apanaskevich and Horak (2008a).
8.3 Morphological Features of Males of Hyalomma Species of Economic and Ecological Importance (Hoogstraal 1956; Walker et al. 2003; Estrada-Pena et al. 2004)
8.3.1 Hyalomma scupense (= H. d. detritum, H. d. scupense)
Morphology of male tick (Fig. 8.1): Male ticks: Scutum is smooth, shiny and plain-coloured. Punctations are very few, large, shallow and scattered. Lateral lines (marginal grooves) are distinct up to a third of the scutum and extends beyond the mid-length of the scutum (may be obscured with indistinct punctations). The median groove is distinct and paramedian grooves are broad and distinct, with lines of rough surface in them. Scutum in the posterior region is formed into four raised ridges. Central festoon (parma) is pale coloured. Legs are not ringed (without bands). Subanal shields are small. There is extensive individual and geographical variability in the morphological characters of H. scupense Apanaskevich et al. (2010).
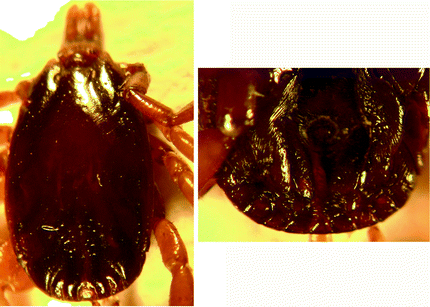

Fig. 8.1
Hyalomma scupense: male dorsal view and male ventral shields
Hosts: Domestic cattle and horses are the most common hosts, but sheep, goats and camels may also be infested. All stages of development feed on the same host species. Adults attach on the inner thighs, udder, scrotum and perineum of cattle.
Distribution: The tick is present in Europe, Middle East, Asia, along the Mediterranean coast of Africa to Algeria and Morocco in the west. It also occurs in north-central Sudan but is not a common tick.
Life cycle: Hyalomma scupense (=H. detritum, Guglielmone et al. 2009) has a two-host life cycle that takes a year to complete. All tick stages feed on livestock. The adults are present during summer, and the larvae and nymphs in autumn. The detached engorged nymphs enter a winter diapause and moult to adults the following summer. Infestation is often associated with barns, stables and sheds, and livestock become infested when they are housed in these structures. The former H. d. scupense is biologically different. It is a one-host species.
8.3.2 Hyalomma rufipes
Morphology (Fig. 8.2): Male ticks: Large, robust, shiny black tick. Scutum is densely, uniformly covered with punctations obscuring lateral lines (grooves). Posterior margin of scutum is evenly rounded with little differentiation. Legs are distinctly annulated.


Fig. 8.2
Hyalomma rufipes: male dorsal view and male ventral shields
Hosts: Adults parasitize particularly the larger species of domestic and wild ungulates. They attach in the hairless perianal region and on the lower perineum and genitalia. The immature stages feed on hares as well as on ground-frequenting birds.
Life cycle: Hyalomma rufipes has a two-host life cycle that takes a year to complete. The adults are most numerous during the early part of the wet season and the immature stages during the dry season.
Distribution: This tick is widely distributed in most of the sub-Saharan African countries and southern Africa. Parasitism of birds by the immature stages undoubtedly contributes to the extensive distribution of this species.
8.3.3 Hyalomma truncatum
Morphology (Fig. 8.3): Male ticks: Lateral (marginal) lines are distinct grooves extending to the eyes. Scutum is dark, smooth and shiny and distinctly concave posteriorly (caudally). This region is densely punctated (contiguous punctations). There is in no parma.
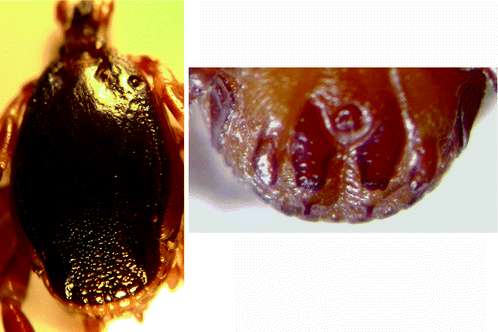

Fig. 8.3
Hyalomma truncatum: male dorsal view and male ventral shields
Hosts: The preferred hosts of the adults are large domestic and wild herbivores. The ticks attach around the anus, on the lower perineum and on the legs, including around the feet. The immature stages feed on hares and on rodents, particularly gerbils.
Distribution: This tick is adapted to dry climates and is widely distributed all over Africa, occupying dry Savannah and high rainfall areas.
Biology and life cycle: Hyalomma truncatum has a two- or three-host life cycle, which normally takes a year to complete. Adults are present in the largest numbers in the late wet summer months and the immature stages in the dry autumn to spring months.
8.3.4 Hyalomma dromedarii
Morphology (Fig. 8.4): Male ticks: Large, 5–7 mm. Scutum wide, smooth shiny and plain colour. Punctations are irregular, few, large and shallow, and rarely small ones occur. Marginal lines (lateral grooves) are very short limited to the posterior third of scutum and deep. Medians and paramedian grooves are deep, narrow with rough surface. The median groove extends from the distinct parma to scutal mid-length. The groove is surrounded on either side by four raised ridges. The subanal shields are prominent, always situated well exterior of the axis of adanal shields.


Fig. 8.4
Hyalomma dromedarii: male dorsal view and male ventral shields; top right fully engorged, bottom unfed specimen
Hosts: The preferred hosts are camels, but cattle, sheep, goats and horses may also be infested. Adults attach on the inner thighs, udder and scrotum of camels. Larvae and nymphs feed on small burrowing animals and on hares, but the nymphs may also infest the same animals as the adults.
Distribution: This tick is common wherever camels occur in the Far, Middle and Near East. It is also present in Mauritania in West Africa and in Morocco, Algeria, Tunisia and Libya in North Africa and is well adapted to an arid and even desert environment. In North eastern and East Africa, it occurs in Sudan, Eritrea and northern, eastern and southern Ethiopia, northern Kenya and north-eastern Uganda.
Biology and life cycle: Hyalomma dromedarii has a two- or a three-host life cycle. The larvae may feed and moult to nymphs on small mammals or hares, and the adults feed on large herbivores. The larvae may feed on small mammal hosts, drop off and moult to nymphs, which can then either attach to other small mammals or feed on the same large animals as the adults. The life cycle appears to be continuous throughout the year.
8.3.5 Hyalomma anatolicum
Morphology (Fig. 8.5): Male ticks: Small ticks are referred to as ‘small Hyalomma’; frail scutum is usually convex, has smooth shiny appearance and is pale in colour. Punctations are small and medium size, shallow, few in numbers and unevenly distributed. Median grooves long and narrow. Scutum in posterior (caudal) area is depressed between two indistinct lateral ridges. Large punctations are found here; surface is rough. Central festoon (parma) is pale coloured, single festoon on each side of the central festoon is of normal shape, separate and not fused (distinctive feature identifying this species from H excavatum). Legs are yellow coloured.
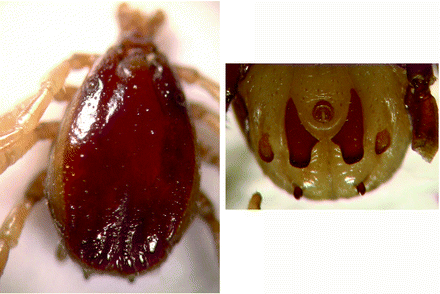

Fig. 8.5
Hyalomma anatolicum: male dorsal view and male ventral shields
Female ticks: Knob-like genital aperture as a distinct bulge protruding from ventral surface; circular outline is characteristic.
Geographical distribution: H. anatolicum ranges from Somalia, northern parts of central Sudan (Southern limit), throughout North Africa, the Middle East, Iran, Indian sub-continent, China, Southern Russia and Turkey, up to southern Europe. This range covers three continents and different climates including desert, semi-desert, Mediterranean and subarctic.
Biology and life cycle: H. anatolicum has a great diversity of diapause mechanisms which regulate its seasonal activity and developmental rhythms in cold climates. Morphogenetic diapause has been found in the nymphal and the engorged female stages of the tick in Palaearctic regions. In North Africa, H. anatolicum adults also undergo behavioural diapause coinciding with shorter and less cold winter season. However, where both seasonal and circadian changes in environment are minimal, as in Sudan, all stages of the tick are active throughout the year. All stages multiply and become a serious pest of livestock in stables and within zero-gazing farming system.
Hosts: H. anatolicum had a mixed two-host and three-host feeding pattern when fed on different host animals. When fed on rabbits, 54–98% of the larvae dropped as engorged nymphs, whereas on guinea pigs and Mongolian gerbils, greater percentage of larvae fed to engorgement and dropped. When H. anatolicum feeds on the normal hosts, e.g. cattle, horses, sheep and goats, a strict three-host cycle is observed.
8.3.6 Hyalomma excavatum
Morphology (Fig. 8.6): Male ticks: Larger ticks strong, shiny dark-brown scutum, with large few and small punctations widely scattered over entire surface, dense in the characteristically posterior (caudal) depression. Lateral lines (marginal grooves), limited to posterior 1/3 but deep and distinct. Posteromedian grooves (paramedian) are indistinct or absent. Scutum in posterior area is concave (depression) between two distinct lateral ridges. These ridges have steep internal margins. Punctations are concentrated here. Central festoon (parma) is pale coloured. The single festoon on each side of the central festoon extends to the anterior; their festoons fuse to form an arch (distinctive feature identifying this species from H. anatolicum). Legs are brown in colour and have distinct pale bands at segments. Ventral shields (plates): adanal strong and broad. Subanals: larger than in H. anatolicum, distinct and dark.


Fig. 8.6
Hyalomma excavatum: male dorsal view and male ventral shields
Hosts, life cycle and biology: This is a two-host tick with the immature stages feed on smaller animals and the adults feed on livestock. It coexists with H. anatolicum but usually occupies the marginal areas. Where H. anatolicum and H. excavatum are sympatric, the former becomes more numerous and uniformly distributed than the latter. This co-existence of the two species has brought some confusion in the naming of H. anatolicum group.
8.3.7 Hyalomma albiparmatum
Morphology (Fig. 8.7): Male ticks: Lateral lines (marginal grooves) are distinct, long and deep and continue to the eye at lines of punctations. Scutum is dark, smooth and shiny with few punctations except posteriorly where it is densely punctate. Posterior region of the scutum is rectangular and concave (similar to H. truncatum). Central festoons (parma) is large whitish or brownish in colour (distinctive feature).


Fig. 8.7
Hyalomma albiparmatum: male dorsal view and male ventral shields
Hosts: The adults feed on domestic animals, mainly cattle, goats and sheep, while the immature stages feed on small animals.
Distribution: The tick is reported from the southern Kenya and northern Tanzania.
8.3.8 Hyalomma marginatum
Morphology (Fig. 8.8): Scutum is dark brown/black and is narrowly elongated, irregularly punctate, dense and large in the distal scapular areas and smaller and shallow and less dense centrally (different from Hyalomma rufipes), and in the posterior area (caudal), it is not strikingly differentiated and posterior margin is bluntly round. Marginal lines (lateral grooves) are long, reaching the eyes, but may be obscured anteriorly. The posteromedian groove reaches the scutal mid-length, being wider and narrow anteriorly. Paramedian grooves are wide near the festoon areas and taper anteriorly. Narrow heavily punctate ridge lies between the paramedian grooves and the lateral grooves. Legs may be entirely reddish (different from Hyalomma turanicum; bright enamelling).


Fig. 8.8
Hyalomma marginatum: male dorsal view and male ventral shields
Hosts, life cycle and biology: The tick undergoes a two-host life cycle with adults infesting cattle and other livestock and the immature stages feed small mammals.
Distribution: The tick is reported from North Africa (Morocco, Algeria and Tunisia) and southern Europe, Turkey and recently reported from Oman and the distribution extends to India.
8.3.9 Hyalomma impressum
Morphology (Fig. 8.9): Recent re-description of adults and larvae was provided by Apanaskevich and Horak (2007). Male ticks: Scutum is regularly densely covered by deep, large punctations obscuring the lateral grooves (marginal lines). The posterior (caudal) region of the scutum is distinctively narrower than the anterior part to form a rectangular outline (distinctive feature). Female ticks: The genital apron is broadly triangular in outline, with anterior bulging ridge and a deeply abruptly depressed narrower posterior button.


Fig. 8.9
Hyalomma impressum: male dorsal view and male ventral shields
Distribution: H. impressum is mainly a West African tick, and it extends its range eastward into western Sudan.
Hosts: The chief hosts of the adult stage are cattle, but it was also reported from cattle and camels.
Biology and life cycle: Not studied.
8.3.10 Hyalomma impeltatum
Morphology (Fig. 8.10): Male ticks: Scutum is dark brown to black, covered by medium-size punctations (few large and shallow). Punctations are larger, deeper and denser on the posterior margin of scutum. Lateral lines (grooves) punctations are clear, extending anteriorly at least to mid-length of scutum and may be obscured by punctations. Subanal shields are large, exterior in position in fed specimen; subanal plates are always situated well exterior of the axis of adanal shields, usually borne on an udder-like swelling extending posterior beyond the body margin. Unfed specimen: subanal shields close to the ventral integument may appear in-line with adanal shields due to the unique tilting of subanal shields in medially directed position.


Fig. 8.10
Hyalomma impeltatum: male dorsal view and male ventral shields
Distribution: The range covers Iran, Middle East, the Mediterranean, Sudan, Eritrea, Somalia, Northern Kenya, northern Tanzania, Chad and West African countries.
Hosts and life cycle: All large domestic animals are hosts of the adult stage, particularly cattle (high infestations were recorded) and camels. The immature stages feed on small animals like rodents, hares and ground birds. Under laboratory conditions, the tick had a three-host life cycle.
8.3.11 Hyalomma aegyptium
Morphology (Fig. 8.11): Male ticks: Subanal shields are present. Coxa I is simple (not deeply divided) with two wide branches. Scutum is smooth, shiny with few large punctations. Festoons unfused. No lateral lines (grooves) or caudal (posterior) depressions.


Fig. 8.11
Hyalomma aegyptium: male dorsal view
Hosts: The tick feeds on tortoise in Mediterranean area.
8.4 Diseases Transmitted by Hyalomma Ticks
Hyalomma species transmit a number of viral, bacterial and parasitic diseases in different parts of the world, making these ticks the economically most important ixodids. The ticks may also act as reservoir of many viral pathogens. Besides disease transmission, certain species of Hyalomma, e.g. H. truncatum, contain toxin in their saliva that causes sweating sickness, an acute dermatitis, in cattle, particularly calves. The attachment of adult ticks to the inter-digital clefts and fetlocks of lambs results in lameness.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


