Fig. 10.1
Light micrograph of unsporulated oocysts of the type Toxoplasma gondii or Isospora from cat feces
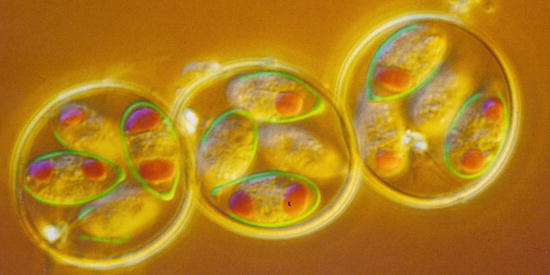
Fig. 10.2
Light micrograph of sporulated Eimeria tenella oocysts from a chicken containing four sporocysts each with two sporozoites
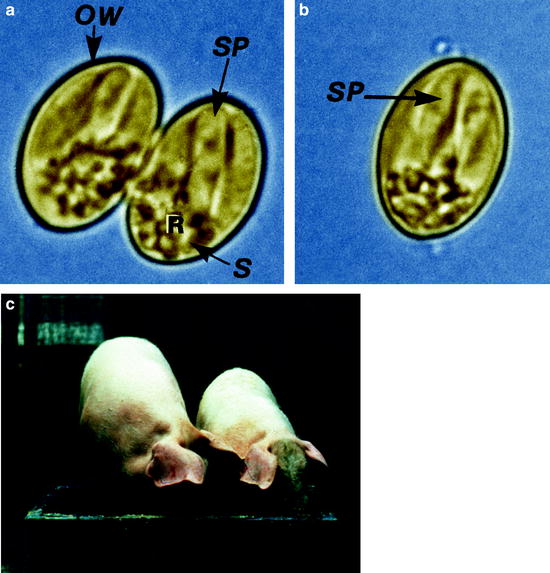
Fig. 10.3
(a) Light micrograph of an sporulated oocyst of Sarcocystis suihominis from human feces containing two sporocysts each with four sporozoites. (b) Single sporocyst. (c) Two pigs of the same age: one with Sarcocystis infection, the other without infection. OW oocyst wall, R residual body, S sporocyst, SP sporozoite
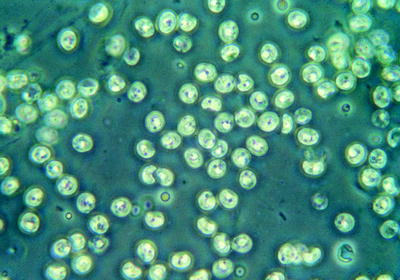
Fig. 10.4
Light micrograph of oocysts of Cryptosporidium sp. from feces of cattle
10.3.2 Trematodes: Sucking Plathyhelminths
Trematodes have a complicated life cycle involving several final hosts. While they are in the intermediate hosts, asexual reproduction occurs, and the sexual stages are formed inside the final hosts. Depending on the organ parasitized, the final hosts (animals, humans) excrete worm eggs via feces, sputum, or urine. However, these eggs—being protected with either a thick or a thin wall—are not infectious if they are taken up immediately by humans or animals that have excreted them, but always need at least one intermediate host for further development. Therefore, these eggs of trematodes are unimportant in studies where flies are investigated as vectors of agents of diseases. Flies only help to propagate trematode eggs within a certain area, and are never involved in a direct human-to-human or animal-to-animal transmission of trematode species (Fig. 10.5).
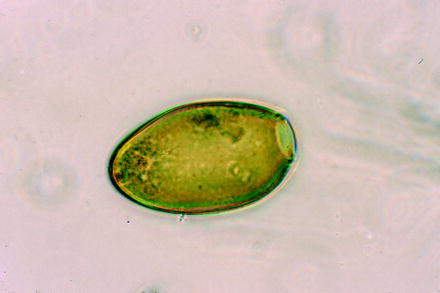

Fig. 10.5
Light micrograph of an egg of the trematode Fasciola hepatica (large liver fluke of ruminants)
10.3.3 Cestodes: Tapeworms
In general, like trematodes, most tapeworms need at least one intermediate host, within which the larva reaches a size that allows it to be transmitted (mostly by feeding on raw or undercooked meat) to a final host (such as humans or predator animals). However, in contrast to trematodes, there are important exceptions among the cestodes. For example, Hymenolepis species and also the human pig-derived tapeworm Taenia solium are able to infest the final host (humans) by means of excreted eggs (Fig. 10.6). In these cases, humans might become intermediate hosts (bearer of larvae) besides their normal activity as final hosts, where the adult tapeworm lives in the intestine and dispatches terminal proglottides that are filled with eggs. In those cases where the proglottides are disrupted inside the intestine or become digested as a consequence of an antitapeworm drug treatment, eggs may release the infectious larvae (oncosphaera or cysticercoid) inside the intestine (Figs. 10.7 and 10.8). In the case of Hymenolepis species, these larvae produce new adult tapeworms, whereas in the case of Taenia solium, a cysticercus may be formed, e.g., in human brain, or a large tissue cyst may develop when humans ingest Echinococcus eggs. If the cysticercus formation unfortunately occurs in the liver or in the brain, the severe symptoms of a hepatic cysticercosis or neurocysticercosis may occur (Mehlhorn 2008, 2012). Therefore, flies may transport eggs/larvae of some tapeworms from human to human and/or animal to animal. Thus, deposition of human feces close to highway parking lots, camping grounds, etc., should be strictly avoided, since feces principally attracts flies, which may become loaded there with tapeworm eggs as described above besides other pathogens.
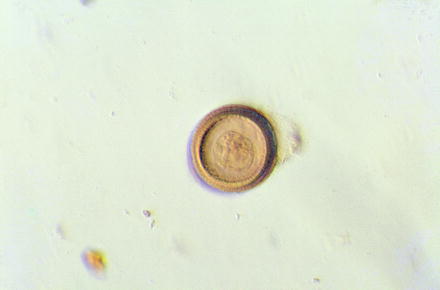
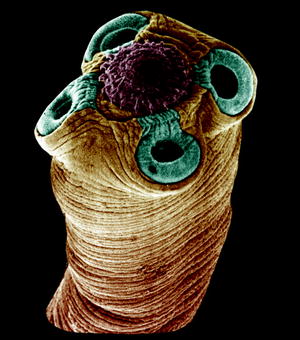
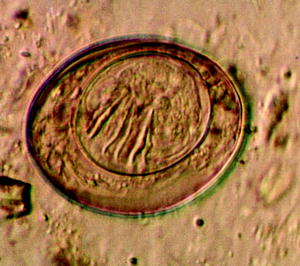

Fig. 10.6
Light micrograph of an egg of the human tapeworm Taenia solium showing its thick outer wall (embryophore)

Fig. 10.7
Scanning electron micrograph of the scolex of a Hymenolepis tapeworm

Fig. 10.8
Light micrograph of the egg of the tapeworm Hymenolepis nana
10.3.4 Nematodes
Only rather few nematodes (roundworms) that infest land-living hosts have a complicated life cycle involving final and intermediate hosts. For example, Trichinella spiralis and the mosquito-transmitted filariae such as Onchocerca volvulus and Wuchereria bancrofti have obligatorily multihost life cycles and thus cannot be transmitted from final host to final host by licking flies, which owing to their peculiarities in transmission never come into contact with the transmissible stage of the nematode. However, a large number of nematodes infesting humans as well their pets and food animals have a direct life cycle which ends with the fecal excretion of very resistant eggs that often have very thick shells (Figs. 10.9–10.11). After temperature-dependent development of a larva inside the eggshell, the egg (or the hatched larva) is ready for transmission, which occurs in general by ingestion of fecally contaminated food of humans or animals. This type of regular transmission of these eggs offers, of course, many possibilities for flies to become mechanical vectors of such eggs. Therefore, it is not astonishing that many nematodes with a worldwide distribution and infections occurring in many millions of hosts (e.g., Ascaris, Ancylostoma, Trichuris) profit from an additional pathway of transmission by licking flies (Anderson 2000; Bramley et al. 1985).

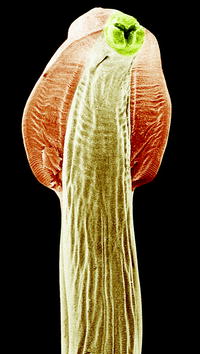
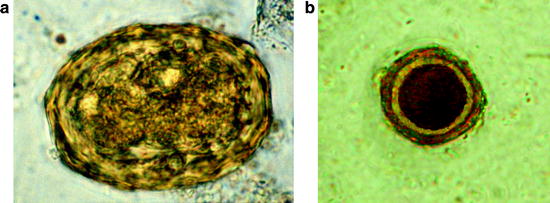

Fig. 10.9
Macrophotograph of adult Toxocara canis worms excreted after treatment

Fig. 10.10
Scanning electron micrograph of the anterior region of the nematode Toxocara canis showing lateral alae (broadenings at the anterior pole)

Fig. 10.11
(a) Light micrograph of a thick-shelled egg of the human roundworm Ascaris lumbricoides. (b) Egg of Toxocara canis found in flies caught at a dog meadow
10.3.5 Mites and Flies
If one looks in the wild especially when capturing insects, it is obvious that many insects are covered by numerous small stages of mites and ticks, which use beetles, dragonflies, butterflies, etc., as means of transportation (phoresy). Some of these “voyagers,” however, even act as parasites, since they suck the hemolymph of their transporters. Thus, it is not astonishing that flies are also targets of such arthropods, which may enter flies when they are sitting on the soil or on animals. If the flies come into contact with larval stages of such arthropods, their transmission to other hosts and/or sites seems likely (Figs. 10.12 and 10.13).



Fig. 10.12
Light micrograph of different developmental stages of the bloodsucking mite Dermanyssus gallinae, some of which had sucked (red) and others had not yet sucked (white)

Fig. 10.13
Photograph of two adult Lucilia flies
10.4 Flies Close to Animal Stables and Downtown Recreation Sites
The main fly species that was found at all stables was Musca domestica, whereas close to the recreation sites Musca domestica was only ranked in fourth place, behind Sarcophaga, Calliphora, and Lucilia species. Only specimens of the genus Calliphora occurred in lower numbers (Gestmann et al. 2012). However, the highest species variation occurred at the dog meadow, where 12 species of flies were caught, headed by Lucilia caesar, Muscina stabulans, and Sarcophaga carnaria, all being attracted by the numerous portions of dog feces. Only single specimens of Musca domestica were found there, reaching less than 0.5% of the total catch (Gestmann et al. 2012). This distribution supported expectations that parasites on those flies caught at different sites would belong to different types of parasites. All species are known to infest humans and to lead to myiasis (Table 10.1).
Table 10.1
Characteristics of the main fly species observed at recreation sites
Species | Size of adults (mm) | Feeding sites | Characteristics |
|---|---|---|---|
Musca domestica | 7–8 | Larvae: omnivorous | Adults: black with gray powder |
Adults: fruit, feces, dead bodies | Males: eyes without contact | ||
Females: posterior of abdomen yellowish | |||
Sarcophaga carnaria | ~14 | Larvae: in earthworms, dead bodies | Adults: light gray; eyes are red |
Adults: fruit juices | Female: deposit larvae instead eggs; enter houses | ||
Lucilia (Phaenicia) sericata | 5–7 | Larvae: necrophagous, in wounds | Adults: greenish metallic; third segment 4 times longer than broad |
Adults: close to humans | |||
Lucilia caesar | 6–8 | Larvae: necrophagous | Adults: greenish metallic |
Adults: in the wild, plant and fecal juices | Males: possess a large, shiny hypopygium | ||
Muscina stabulans | 7–9 | Larvae: saprophagous | Adults: body Musca-like, yellowish legs |
Adults: on fruits, walls, trees, markets | Larvae: spiracle slits widely separated | ||
Calliphora vicina (syn. erythrocephala) | 8–10 | Larvae: feces, wounds | Body bluish shiny |
Adults: close to or in houses | Males: face yellowish brownish | ||
Calliphora vomitoria | 10–14 | Larvae: feces, wounds | Body bluish, head reddish, fly sound dense |
Adults: rare in houses | |||
Tachina fera | 12–15 | Larvae: in larvae of butterflies | Head yellow, thorax black, abdomen yellow |
Adults: on flowers | |||
Fannia canicularis | 4–6 | Larvae: feces, decaying material | Adults are covered with grey hair, thorax brownish-grey with three brown stripes |
Adults: toilets, dung | |||
Pollenia angustigena | 5–12 | Larvae: in earthworms | Adults appear blackish with square dots on the abdomen |
Pollenia rudis | Adults: overwinter in houses |
10.5 Parasites Found on Flies Caught in the Wild
In total, 22 different parasites were found in or on flies caught close to stables or at the dog meadow. They belonged to the groups of protozoa (with six different coccidians, Giardia lamblia, Fig. 10.14, Herpetomonas muscarum, Balantidium coli), nematodes (with five defined and several undefined species), and three determined arachnid species in addition to several undetermined ones. In the following sections, more details on the flies caught and their parasitic load are given (Fig. 10.15).
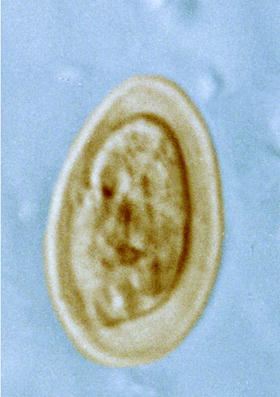
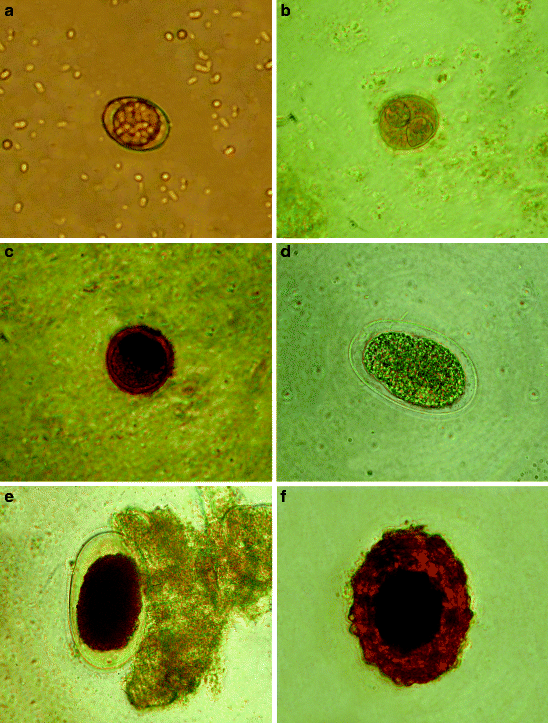

Fig. 10.14
Type of Giardia cysts seen in feces

Fig. 10.15
Micrographs of parasites seen on or inside flies caught in pigpens. (a, b) Unsporulated and sporulated oocysts of Isospora suis. (c) Balantidium coli cyst. (d–f) Eggs of nematodes
10.5.1 Parasitic Load of Flies at Pigpens
The results of the examination of 593 flies caught close to a pigpen are given in Table 10.2.
Table 10.2
The results of the examination of 593 flies caught close to a pigpen
Parasite | Stage | Number of flies testing positive | Number of parasites detected | |
|---|---|---|---|---|
IN | EXO | |||
Giardia lamblia | Trophozoite | 2 | 100 | – |
Cyst | 2 | – | 8 | |
Eimeria perminuta | Oocyst | 2 | 8 | – |
Isospora suis | Oocyst | 7 | 18 | 70 |
Balantidium coli | Cyst | 13 | 16 | – |
Ascaris suum | Egg | 12 | 4 | 16
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|