Fig. 13.1
Scanning electron micrograph (SEM) of the lateral view of a cat flea (Ctenocephalides felis) attached at a hair
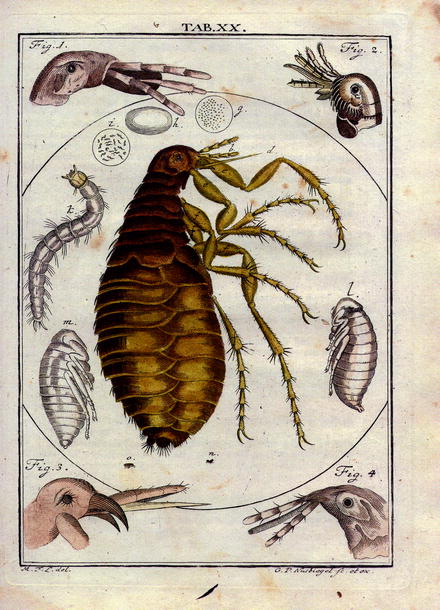
Fig. 13.2
Reproduction of a plate picturing the knowledge on fleas in the year 1760: facts and phantasy from: Martin Groben Ledermüllers: Microscopical mental and eye amusement with colors according to nature. Printer C. de Launoy at Nürnberg (Germany) (in German)
At first, humans had to recognize that fleas are nasty bloodsuckers and that the wounds at the biting sites reacted due to the injection of saliva with the formation of papulae and intense itching that lasts for days (Fig. 13.3a). In case of bacterial superinfections due to scratching at these biting sites, severe inflammations or even sepsis may occur (Fig. 13.3b). In addition, some flea species, e.g., the females of the sand flea Tunga penetrans or of the chicken flea Echidnophaga gallinacea, may introduce directly diseases by entering totally respectively partially the skin of their hosts (Figs. 13.4–13.6).
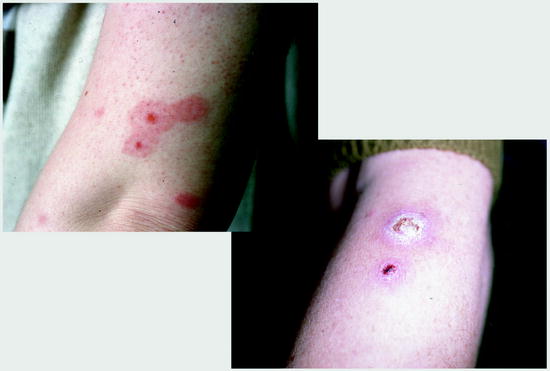
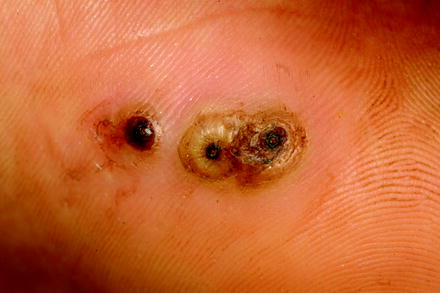
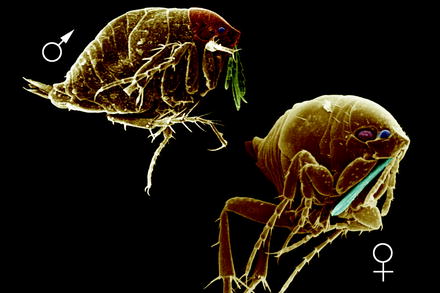
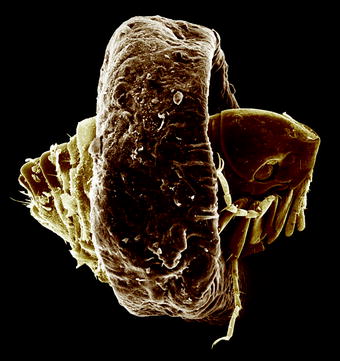

Fig. 13.3
Flea bites without (left) and with bacterial superinfection. Flea bites often occur in rows

Fig. 13.4
Three females of Tunga penetrans just penetrated into skin

Fig. 13.5
SEM of female and male specimens of Tunga penetrans

Fig. 13.6
SEM of a female T. penetrans from skin showing the swollen midregion of the abdomen
Since most of the specimens of more than 2,500 described species are not very host specific and may even change their hosts daily, it is not astonishing that during evolution they became vectors of different agents of diseases, which occur in the bodies of their hosts. Therefore, flea infestations of humans—especially of persons who live in camps and close neighborhoods with rats and murine species—have to be taken into serious consideration, since the outbreak of severe diseases (e.g., plague, rickettsiosis, bartonellosis, etc.) may have disastrous consequences not only for local people but also—after immigration—for inhabitants of other continents. This danger of a transport of emerging diseases from one continent to another increases daily, since the “heat” of the worldwide globalization is not yet under control due to the fact that one end of the world is just one jet-day away from the other. Thus, flea infestations of humans and their pet animals are no longer neglectable nasty events of itching but may produce health-threatening scenarios.
13.2 Why Are Fleas So Successful Survivors?
During evolution, fleas had to overcome many obstacles, which could threaten their survival, and spreading into constantly changing climates, since the “good old earth” exposed them to many warm and icy periods at rather short intervals during several millions of years. Therefore, the fleas developed the following skills (Figs. 13.7–13.11):

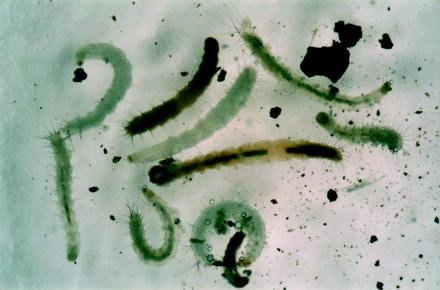
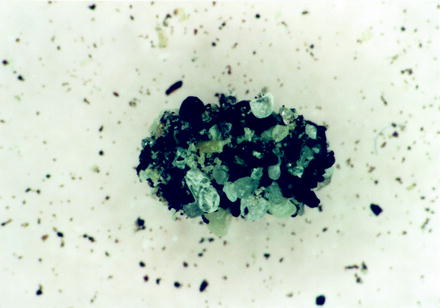
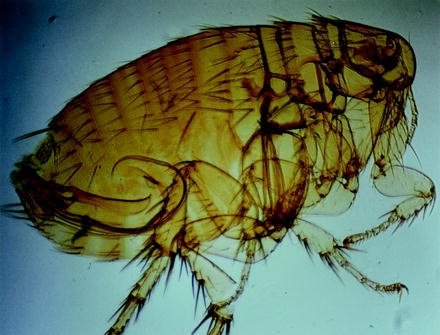


Fig. 13.7
Light micrograph of a cat flea egg on a dead adult inside detritus of a dwelling

Fig. 13.8
Differently aged larvae of the cat flea, some of which had fed dried blood

Fig. 13.9
Pupal cocoon of a cat flea covered by small stony particles

Fig. 13.10
Light micrograph of a male of the human flea Pulex irritans. Note that there are no bristles along the head. The protrudable copulation apparatus is visible in the abdomen of this specimen, which was made transparent

Fig. 13.11
SEM of Pulex irritans, the so-called human flea
1.
Flea ancestors started to suck blood at the tiny warm-blooded precursors of the mammals of our days (after the disappearance of the dinos).
2.
This protein-rich food allowed a rather quick individual development so that fleas may complete their life cycle (from egg via three larvae, one pupa, and ending as male or female adult) under good, warm conditions (27°C and >50% relative humidity) within 3–4 weeks.
3.
This admirable efficiency is supported by the capability of the females to lay (in the case of Ctenocephalides) up to 40 of the 0.5 mm-long whitish eggs per day while sucking every 3–4 h a total of about 14 μl blood per day representing several times of their body weight.
4.
The females and males do not suck blood to cover only their own requirements, but they excrete so much superfluous blood in their feces so that they may nourish crowds of larvae, which then appear with a dense intestine (Fig. 13.8).
5.
Again, this energy-rich food allows the adults a survival period of at least 5–6 months in their pupal cocoon prior to hatch, if there is no shaking signal that announces the arrival of a potential blood donor.
6.
Once hatched from the pupal cover, the male and female adults start feeding at the next warm-blooded host, since a better one might not come. Therefore, most fleas accept a very broad spectrum of hosts.
7.
8.
This persistence of fleas for a self-selected long period on a host also helps to distribute fleas in a given biotope, since all warm-blooded animals have to roam often for long distances in order to find appropriate food.
9.
The fact that at first the eggs glue at the hair of hosts and drop down to earth after some days when the egg shell has become dry also helps that flea stages are differently distributed in a biotope and are able to find other hosts.
10.
The high body temperatures along the hair—or feather—covered body surface of the hosts of fleas guarantee high survival chances.
11.
If hosts of fleas leave their nests or homes even for months, these surroundings protect fleas until new inhabitants enter these “domiciles.”
12.
The fact that fleas are not host specific helps that they may obtain their food much easier than host-specific bloodsuckers.
13.
Since the adults live hidden in the hair respectively among the feathers of their host, their number is not reduced by enemies.
Thus, nature has gifted fleas, which can be differentiated (among other criteria) by the bristles at the head and/or pronotal segment (Figs. 13.12 and 13.13) so perfectly, that under optimal conditions very often a mass production of fleas may occur, which guarantees survival of these perfect parasites—of course surely not to the pleasure and welfare of their hosts.
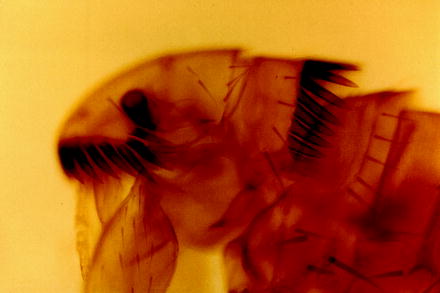


Fig. 13.12
Light micrograph of the anterior portion of the cat flea (C. felis) showing the typical bristles

Fig. 13.13
SEM of the head of the cat flea
13.3 Why Are Fleas Successful Vectors of Agents of Diseases?
There are several crucial conditions for the successful transmission of agents of disease to humans:
1.
The presence of sufficient agents of disease in the host spectrum of fleas is highly needed in a given biotope, where fleas may switch from wild animals to humans.
2.
There must be a common transition of potentially infected fleas to humans, i.e., there must be a broad contact zone between flea-bearing animals and humans.
3.
The survival (and even better) reproduction of such agents of disease in the mouthparts or in the upper intestinal tract of the fleas must be possible.
4.
The presence of a large number of fleas that attack different warm-blooded hosts and switch from one host to another is very helpful, since many different hosts might be infected by previously ingested pathogens.
All these conditions are fulfilled in the case of fleas:
1.
They are obligatory blood feeders.
2.
They suck blood several times per day (potentially at different hosts).
3.
They suck large amounts of blood so that they can take up large quantities of pathogens.
4.
They leave their hosts from time to time (e.g., in the nest of birds and/or rodents) and have the chance to infest other hosts.
5.
They are not host specific and accept a broad spectrum of different warm-blooded hosts.
6.
Fleas have developed a peculiar behavior while sucking blood, which is supported by the construction of their stomach system. The latter consists of rather stiff, not enlargeable, chitinous bristles containing prestomach and a larger posterior enlargeable portion (Fig. 13.14). Since fleas are hasty blood suckers (ingesting large amounts of blood within minutes), the transport of the masses of blood may be blocked before the prestomach, introducing a reaction called regurgitation (i.e., the vomiting of blood into the bite site). This regurgitated blood represents often a mixture of blood of potentially different hosts, in case a preceding meal on a host was interrupted and the flea switched to another host afterward.
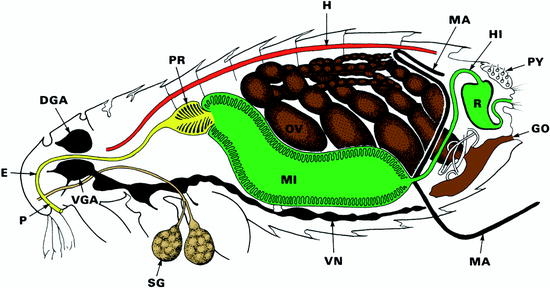

Fig. 13.14
Diagrammatic representation of the lateral view of a female flea. DGA dorsal ganglion (brain), E esophagus, GO genital opening, H heart, HI hind gut, M Malpighi channel (= excretory system), MI midgut, P pharynx, PR prestomach, OV ovary, PY pygidial plate = sensory bristle system, R rectum, SG salivary gland, VGA ventral brain ganglion, VN ventral nerve strand
7.
Some agents of disease (e.g., plague bacteria) attach at the wall of the prestomach, reproduce there by repeated fissions, and thus may block—at least partially—the further inner transportation of the ingested blood. This phenomenon introduces regular common regurgitation of blood and bacteria into the wound.
8.
Fleas suck hastily, a phenomenon which also may lead to regurgitation even if bacteria do not block the entrance of the stomach.
All these biological, morphological, and behavioral peculiarities of fleas listed above are favorable for the transmission of microorganisms such as typical bacteria, Rickettsiales, mycoplasms, and/or viruses. However, fleas are also known as intermediate hosts of tapeworms and/or nematodes. In these cases, the flea larvae are orally infected, when feeding worm eggs or larvae, which then are stored in the larval bodies and finally are transferred (via pupal stage) into the adults. If larvae or adults are cracked and eaten by the final hosts of these worms (e.g., cats, dogs, human kids) in the case they get into mouth contact with fleas or portions of them (in the hair of companion animals), the worm reaches maturity in these hosts.
However, although in principle each flea species might transmit practically any microorganism and/or parasite that has entered the bloodstream of any host, there are proven significant differences between different flea species with respect to their efficacy rate in transmitting a given agent of disease. This evolved transmission ability surely depended on the duration and frequency of the exposition that occurred between vectors and infected hosts. Therefore, the occurrence and recently visible or increasing successful transmission of a microorganism and/or a parasite in a given biotope depends on the following criteria:
1.
Pathogen acquisition efficacy of the individual members of a certain flea species.
2.
Vector efficiency of this flea species.
3.
Length of the period needed until fleas may transmit an once ingested agent of disease (being called extrinsic incubation period (EIP)).
4.
Reproduction activity of the agents of disease inside the flea leading to a possible blocking of the upper intestinal system and thus initiating repeated regurgitation with introduction of the pathogens into the new host.
5.
Host change behavior of the flea species. High host change rates support intensely spreading of pathogens within a biotope and its inhabitants.
Considering all these conditions, Eisen and Gage (2012) showed in their excellent review that among the 30 experimentally tested flea species which had been previously confirmed as vectors of Yersinia pestis in North America, only a few were really good vectors. Thus, the worldwide-known vector species Xenopsylla cheopis (Fig. 13.15) showed in most studies a pathogen acquisition efficacy higher than 70%, reaching often up to 100%, a vector efficiency rate of around 30–70%, an EIC-period mostly of only 5–20 days, and in many cases a blocking of the intestinal tract in more than 70% of the cases (Douglas and Wheeler 1943; Eisen et al. 2007a, b; Eskey and Haas 1940; Wilder et al. 2008). Other flea species were much less efficient in the transmission of Y. pestis. As an example, the typical human flea Pulex irritans (Figs. 13.10 and 13.11), that is also found on many mammals, reaches a pathogen acquisition efficiency of around 31%; however, none of 57 fleas tested was able to transmit, which surely depended on the fact that only 2% of the fleas showed a blockage of the upper intestinal system (Burroughs 1947). The same author showed that the poultry flea Echidnophaga gallinacea had a pathogen acquisition efficiency of 80% and a vector efficiency of 23%, apparently due to the fact that 23% of the fleas had a blocked intestinal system. The cat flea (Ctenocephalides felis), which is the most common flea (around 80%) on humans, dogs, and cats in Europe, showed an extreme high pathogen acquisition efficiency of 85%; however, practically no vector efficiency and blockage of the intestinal tract occurred (Wheeler and Douglas 1945a, b; Eisen et al. 2008). On the other hand, the flea species of ground squirrels (Oropsylla montana) reached even better values than the internationally well-known Xenopsylla cheopis strains, when showing in recent experiments a pathogen acquisition efficiency of 100% and low vector efficiency rates of about 10%, since there was no blocking of the stomach inside the fleas. Nevertheless, the final total transmission effect was good since there was an early-phase transmission (Eisen et al. 2008, 2009a, b).
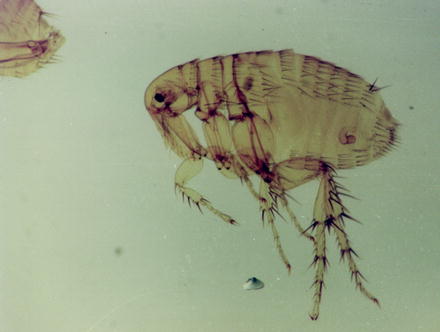

Fig. 13.15
Light micrograph of a female Xenopsylla cheopis flea. From the female system, only the chitinous spermatheca (where the sperms are stored) is retained after the preparation procedure
These different capacities of the different flea species point to some facts, which have always to be considered, when evaluating a transmission risk in a certain region of the world:
1.
Fleas can potentially transmit all pathogens contained in blood.
2.
However, the occurrence of such pathogens in blood will not lead to an effective transmission in all cases, but on the other hand, single cases of transmission are never excluded and often only small amounts of pathogens are sufficient to establish an infection.
3.
Outbreaks of disease will only occur in cases where large numbers of hosts get into close contact with large amounts of possibly infected fleas with a high vector capacity.
4.
Blockage of the anterior intestinal portions of fleas by pathogens is not needed to establish an infection of humans but is surely helpful (Fig. 13.14).
5.
Thus, the best measure to avoid flea-borne transmission of pathogens is to keep away masses of flea bearers (e.g., rodents, wild dogs, etc.) from humans according to the simple rule: no mammals as pathogen carriers + no fleas = no potential transmission.
6.
However, due to the crowding of humans and rodents at places (i.e., in refugee camps, at playgrounds, etc.) and as a consequence of the warming up of the climate, several emerging diseases due to flea transmission will surely occur in many places of the world.
13.4 Which Factors May Support the Increasing Vectorship of Fleas?
Recent reports (Friggens and Beier 2010) confirm what has been suggested for a long time (Patz et al. 2000; Deem et al. 2001; Daszak et al. 2001; Wilcox and Gubler 2005; Wilcox and Colwell 2005; Koontz and Daszak 2005; Crowl et al. 2008) that human influence (anthropogenic habitat disturbances) disrupts ecosystem processes and supports the relevant zoonotic disease dynamics. These disturbances are mainly based on the growth of human population, increase of urbanization, agricultural and forestry intensification (monocultures), and encroachment into wild (natural) areas. These factors led to severe changes and to considerable decrease of the species diversity of small mammals in a given biotope (Tikhonova et al. 2006). If one had expected that this fact would lead to a decrease of the number of fleas, it turned out to be wrong. Egoscue (1976) already showed that low host diversity often favored some common flea host species and thus led to an increase of flea species and their individual number. This was confirmed by the study of Friggens and Beier (2010), who tried to understand how fleas and flea-borne diseases might be influenced by human disturbances. They investigated the biotope flea community dynamics and flea host utilization patterns in relation of the intensity of disturbance. In their analysis of 70 small mammal communities, it turned out that human disturbance led to a decrease of host diversity but to an increase of flea infestation on small mammal hosts. They concluded that all important conditions were given—even under low disturbances—that would support an increase of transmission of flea-borne diseases, if their agents have once entered the bodies of these hosts.
Thus, flea-borne diseases must be added to the group of emerging diseases, even if they are not imported from abroad. In the latter cases, they would be described as reemerging diseases. These dangers are thus supported by three main factors:
1.
Human-bound change of local biotopes as described above.
2.
3.
Globalization, which brings people and animals (including fleas) from all over the world to special habitats in new countries. Thus, a transfer of agents of disease needs only a few hours and may lead to severe outbreaks (Romi 2010).
13.5 Fleas as Vectors of Zoonotic Agents of Disease
Fleas were detected as possible vectors of agents of disease as early as mosquitoes were shown to be vectors of malaria. In both cases, the agents of the disease (e.g., plague bacteria respectively malaria parasites) had been described earlier (literature see Grüntzig and Mehlhorn 2005, 2010a, b). It was the French scientist Paul-Louis Simond who described the capacity of the tropical rat flea Xenopsylla cheopis (Greek: xenos = strange; psylla = flea; cheops = famous Egyptian pharaoh 2,500 B.C.) to transmit the simultaneously by the Swiss scientist André Yersin and the Japanese Shibasaburo Kitasato 1894 discovered bacterium being described today as Yersinia pestis (Fig. 13.16). In the last century, it was shown that these bacteria might be transmitted by many flea species. However, the vector efficacy is highest in X. cheopis and a special rodent flea species (e.g., Oropsylla montana). Studies with fleas showed that local and ubiquitous species of fleas may transport and transmit a broad spectrum of microorganisms and parasites (Table 13.1).
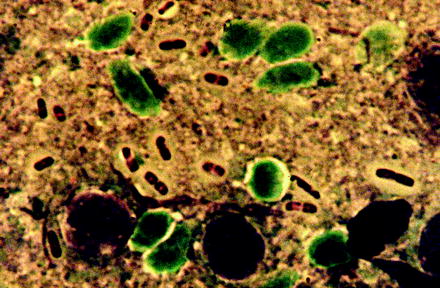

Fig. 13.16
Smear preparation of a fluid containing several plague bacteria (Yersinia pestis), which had been obtained from an open bubonic plague spot
Table 13.1
Medically important flea species around the world (selection)
Species/geographical distribution | Length (mm) | Main hosts/humans | Transmitted pathogens |
|---|---|---|---|
Ceratophyllus gallinae/E | m 3.0 | Chickens, turkeys, dogs, cats, humans | Mechanically many pathogens, Omsk hemorrhagic fever |
f 3.5 | |||
Ctenocephalides canis/worldwide | m 2.5 | Dogs, humans | Larvae of cestodes (Dipylidium, Hymenolepis), Yersinia pestis, Mycoplasma (formerly, Haemobartonella) haemofelis |
f 3.5 | |||
C. felis/worldwide | m 2.5 | Cats, humans | Larvae of cestodes (Dipylidium, Hymenolepis, Dipetalonema), Yersinia pestis, viruses, R. felis, R. typhi |
f 3.0 | |||
Echidnophaga gallinacea/worldwide | m 2.0 | Chickens, dogs, cats, pigs, humans | Bacteria, Yersinia pestis |
f 2.5 | |||
Leptopsylla segnis/E, EA | m 1.6 | Murine rodents | Mechanically many pathogens, cestodes |
f 1.8 | |||
Nosophyllus segnis/E | m 1.8 | Rats, humans | Yersinia pestis, other bacteria, erysipeloid |
f 2.0 | |||
Pulex irritans/worldwide | m 2–2.5 | Humans, domestic animals | Yersinia pestis, erysipeloid, larvae of cestodes |
f 4 | |||
Spilopsyllus cuniculi/E | m 1.6 | Rabbits, humans | Myxomatosis virus, Francisella tularensis |
f 20 | |||
Tunga penetrans/E
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|