Chapter 13. The Glaucomas
CHAPTER CONTENTS
The canine glaucomas423
Goniodysgenesis associated glaucoma (primary glaucoma, acute primary angle-closure glaucoma, primary open-angle closed-cleft glaucoma) 423
Sequential changes in the drainage angle following acute IOP elevation in goniodysgenesis-related glaucoma 425
Chronic changes 425
Sequential changes in the retina following acute IOP elevation in goniodysgenesis-related glaucoma 425
Chronic changes 425
Sequential changes in the optic nerve following acute IOP elevation in goniodysgenesis-related glaucoma 425
Chronic changes 426
Canine primary open-angle glaucoma (POAG) 428
Lens luxation glaucoma 429
Neovascular glaucoma 429
Glaucoma in canine ocular melanosis 438
Multiple thin-walled iridociliary cysts, pigmentary uveitis in Golden Retrievers 438
Glaucoma associated with neoplasia in dogs 438
The feline glaucomas440
Some general features of feline glaucoma compared to canine glaucoma 440
The majority of cases of feline glaucoma are secondary to other ocular disease 440
Lymphoplasmacytic uveitis 440
Glaucoma associated with feline diffuse iris melanoma 441
Spontaneous primary glaucoma is relatively rare in the cat 443
The equine glaucomas446
GENERAL CONSIDERATIONS
Structure and function of the normal aqueous outflow pathways (Fig. 13.1)
Maintenance of a physiologic intraocular pressure relies on a delicate equilibrium between aqueous humor production and outflow.
• Aqueous is produced by the ciliary processes, by a combination of mechanisms including diffusion, ultrafiltration and active secretion, into the posterior chamber
• From the posterior chamber, aqueous flows through the pupil into the anterior chamber, then enters the ciliary cleft via spaces within the pectinate ligament, which spans the irido-corneal angle (ICA)
• Within the ciliary cleft, aqueous humor percolates through spaces between collagenous beams of the ciliary cleft, then the corneoscleral trabecular meshwork (TM). The corneoscleral TM is embedded in the sclera and closely associated with the collector vessels of the angular aqueous plexus, which are analogous to the annular ‘Schlemm’s canal’ of primates
• Fluid is then transported by a pressure dependent mechanism, via the vacuolating endothelium of the angular aqueous plexus, to the radially oriented collector channels of the intrascleral venous plexus. From there, aqueous passes into the scleral and choroidal veins
• In addition to pressure dependent ‘conventional’ drainage via the trabecular meshwork into intrascleral and episcleral veins, aqueous can also percolate via a posterior ‘uveoscleral’ route through the ciliary body interstitium to the suprachoroidal space and vortex veins. Uveoscleral outflow has been estimated to account for about 3% of aqueous outflow in normal cats, and about 15% of aqueous outflow in normal dogs.
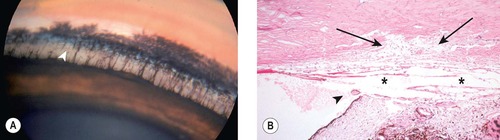 |
| Figure 13.1 Normal canine irido-corneal angle structures. (A) Beagle cross, 4 years old: the pectinate ligaments span the angle from the base of this iris to Descemet’s membrane. The superficial and deep pigmented bands are present. The uveal trabeculae (arrow) are easily seen within the ciliary cleft. (B) Photomicrograph of the normal canine irido-corneal angle showing the primary pectinate ligament (arrowhead), ciliary cleft (*) and corneoscleral trabecular meshwork (arrows). |
The glaucomas: significance and general principles
Definition
The glaucomas represent a large, diverse group of pressure dependant neurodegenerative disorders, that all result in loss of normal function and integrity of the retinal ganglion cells and their axons in the optic nerve and ultimately lead to loss of vision.
• In veterinary patients, the single most consistently recognized feature of all glaucomas characterized to date is elevation in intraocular pressure (IOP).
Significance to a mail-in ocular pathology service
• Glaucoma leads to loss of vision, with varying degrees of ocular pain or discomfort. Despite advances in medical and surgical therapy, the prognosis for restoration and maintenance of vision remains quite poor. Given this relatively poor prognosis for vision, and that therapy can be costly for control of a disease that is often a source of significant discomfort, glaucoma is a common reason for enucleation and subsequent submission of the globe to a pathology service
• 41% of canine submissions to COPLOW have glaucoma as a part of the syndrome
• 29% of feline submissions to COPLOW have glaucoma as a part of the syndrome
• Glaucoma is probably under-represented in equine submissions. The condition often goes clinically undiagnosed in horses as tonometry is less frequently performed in this species and the pain of glaucoma is not manifest as acutely as it is in dogs.
Non-specific changes in ocular tissues associated with elevated intraocular pressure (Fig. 13.2)
Many of the changes listed below can represent either a cause or an effect of glaucoma. It is often difficult to determine which, with confidence.
• Cornea and sclera
▪ Corneal edema
▪ Descemet’s streaks (Haab’s striae) associated with tears or splits in Descemet’s membrane
▪ Corneal vascularization
▪ Scleral atrophy at the limbus
▪ Thinning, stretching and enlargement of the globe (buphthalmos)
– This is more commonly seen in animals than in humans with glaucoma
– Globe enlargement is more dramatic in young animals and children than in adults
▪ Equatorial and limbal staphyloma
• Uvea
▪ Collapse of the ciliary cleft
▪ Atrophy of the corneoscleral trabecular meshwork
▪ Iris atrophy
▪ Ciliary body atrophy
▪ Decreased choroidal perfusion
• Lens
▪ Cataract
▪ Lens subluxation or luxation
• Neurosensory retina
▪ Loss of retinal ganglion cells (all species)
▪ Full thickness retinal atrophy and gliosis, dogs only
▪ Less atrophy of the superior (tapetal retina relative to the inferior (non-tapetal) retina, dogs only
• Optic nerve
▪ Necrosis is an early feature, followed by malacia, gliosis and the formation of a deep cup. In species other than the dog the development of gliosis and cupping follows a less precipitous course
▪ In glaucomatous optic neuropathy, there is a loss of large diameter optic nerve axons.
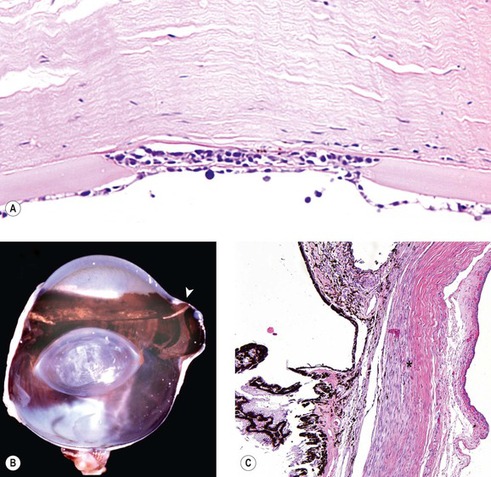 |
| Figure 13.2 Non-specific morphologic features of glaucoma (A) Photomicrograph of a canine cornea showing Haab’s stria in glaucoma (B) Gross photograph of a dog eye showing an acquired staphyloma at the limbus (arrow). (C) Photomicrograph of the irido-corneal angle and the limbal sclera from a dog with glaucoma showing a loss of collagen associated with scleral atrophy (*). |
Underlying pathogenesis
It can be challenging for the pathologist to differentiate primary and secondary changes and suggest the underlying pathogenesis, in the absence of an informative clinical history. For example, lens subluxation may lead to, or result from, glaucoma. Consideration of breed predispositions may increase the index of suspicion for either primary or secondary causes of glaucoma.
Critical events in the pathogenesis of the glaucomas
Many of the critical events in the pathogenesis of the glaucomas remain poorly understood including the following:
• The precise mechanism responsible for the control and regulation of intraocular pressure, particularly at the level of aqueous outflow, is incompletely understood in both normal and glaucomatous animals.
Mechanisms for IOP elevation
• The key mechanisms for IOP elevation are therefore:
▪ Reduction in capacity for aqueous outflow, and/or
▪ Increase in episcleral venous pressure (i.e. ‘back pressure’)
• Reduction in the capacity for aqueous outflow may be:
▪ Primary (goniodysgenesis):
– Due to an inherent abnormality in the aqueous outflow pathways
○ It is worth emphasizing that the cause of increased resistance in the aqueous outflow pathway in goniodysgenesis is not yet known. The possibility that aqueous outflow obstruction might actually prove to be secondary and not primary cannot be discounted
– Primary glaucoma is fairly common in certain breeds of dog
– Primary glaucoma is seldom encountered in other domestic species
▪ Secondary to other ocular or systemic disease processes, as a relatively frequent complication of:
– Uveitis
– Synechiae
– Pre-iridal fibrovascular membrane
– Cataract
– Lens luxation
– Intraocular neoplasia
– Intraocular hemorrhage
– Retinal detachment
• Increase in episcleral venous pressure may occur:
▪ In animals with orbital space occupying lesions
▪ Transiently, as a result of inappropriate restraint restricting jugular blood flow, including tight collars
• Mechanisms for aqueous outflow obstruction:
▪ Pupil block
– Anterior lens luxation, anterior vitreous prolapse, intumescent cataract and extensive posterior synechiae are all relatively common causes of pupil block glaucoma
– Flow of aqueous humour from the posterior chamber to the anterior chamber is obstructed at the level of pupil and aqueous is therefore unable to exit through the conventional outflow pathways via the irido-corneal angle and ciliary cleft
– Elevation in pressure within the posterior chamber leads to forward displacement of the iris, collapse of the ciliary cleft, narrowing of the irido-corneal angle and a shallow anterior chamber. The anterior chamber may be diffusely shallow, or may vary in depth as in iris bombé.
▪ Angle closure
– Alternatively, the iris may be ‘pulled forward’, as occurs in association with contracture of pre-iridal fibrovascular membranes (PIFVM), in a mechanism that can ultimately lead to extensive peripheral anterior synechiae
– Obstruction of the trabecular meshwork and/or obliteration of the ciliary cleft by cellular infiltrates or debris may lead to the development of glaucoma secondary to neoplasia, uveitis and intraocular hemorrhage
• Clinical and pathological nomenclature: open-angle versus closed-angle
▪ Traditionally, veterinary ophthalmologists have tended to classify glaucomas clinically, on the basis of gonioscopic findings, as either ‘open-angle’ or ‘closed-angle’. In reality, what we are really referring to is not simply the irido-corneal angle (ICA), but the opening to the ciliary cleft
▪ In contrast, veterinary pathologists have tended to classify many of the primary glaucomas as ‘open-angle, closed-cleft’. This has led to confusing discrepancies in the clinical and histopathological classification of the canine and feline glaucomas
▪ Until relatively recently our ability to visualize and clinically characterize the ciliary cleft in vivo was extremely limited. Refined imaging techniques, such as ultrasound biomicroscopy (UBM), with probe frequencies around 50 MHz, and high resolution ultrasonography (HRUS), with probe frequencies around 20 MHz, offer us a greater appreciation for the dynamic changes that take place within the aqueous outflow pathways in our glaucomatous patients. These new technologies should facilitate the harmonization of clinical and pathological classification schemes as well as improve our insight into the pathogenic mechanisms involved in each individual patient
Because of the confusion, in this chapter we will avoid the use of this terminology altogether.
Mechanisms of optic nerve and retinal damage
The mechanisms of optic nerve and retinal damage in the glaucomas remain subject to debate. Proposed mechanisms include the following:
• Elevated intraocular pressure leads to deformation of the optic nerve axons at the lamina cribrosa and the blockage of anterograde and retrograde axonal transport which, in turn, leads to the death of retinal ganglion cells. Cell death is due, at least in part, to an interruption in the supply of neurotrophic factors from the CNS
• Elevated intraocular pressure leads to deformation of the lamina cribrosa, leading to a decrease in vascular perfusion of the optic nerve head microcirculation, that results in a loss of axonal integrity and subsequent death of retinal ganglion cells
• Elevated intraocular pressure leads to tissue infarction and subsequent gliosis
• Retinal ganglion cell loss may also be due to the pathologic release of glutamate from within the damaged retina, which initiates the process of apoptosis by a mechanism termed ‘excitotoxicity’
• Decreased choroidal perfusion causes a segmental retinal degeneration affecting both inner and outer retina
• Recent evidence suggests that different mechanisms may be involved in the death of the retinal ganglion cell bodies, axons and dendritic trees.
Mechanisms of retinal and optic nerve degeneration
The relevance of the various proposed mechanisms of retinal and optic nerve degeneration to the spontaneous glaucomas of dogs and cats:
Comparative Comments
• Any or all of these proposed mechanisms might be in play, depending on the species and also the underlying pathophysiology of the different glaucoma syndromes in dogs and cats
• The pathogenic mechanisms in play, and their relative importance, appear to differ considerably between glaucomatous dogs, cats and humans
▪ In the dog, the changes in the retina and the optic nerve head suggest that abnormal vascular perfusion and ischemia are major factors in the pathogenesis of optic nerve and retinal damage encountered in glaucoma syndromes. Severe damage to the inner and outer retina and optic nerve can be observed in dogs at a very acute stage of disease. In acute congestive canine glaucoma, where IOP is frequently >50 mmHg, there is evidence to support an important role for ischemia in cell death within both the inner and outer retina. Breakdown in the blood-ocular barrier and inflammation may also contribute to retinal degeneration
▪ In the cat, the retinal disease is more restricted to the ganglion cell layer, and optic nerve necrosis and malacia is less common.
Comparative Comments
As is the case in other animal species, ‘glaucoma’ in human ophthalmology is generally considered a generic term for a common group of ocular diseases that, if untreated, can cause an irreversible loss of visual function. The common underlying factor in all forms of glaucoma is an inappropriate intraocular pressure, which is associated with damage to the retina and optic nerve head.
Glaucoma in humans is usually divided into five main categories:
1. Congenital glaucoma
2. Primary open-angle glaucoma
3. Primary angle-closure glaucoma
4. Secondary open-angle glaucoma
5. Secondary closed-angle glaucoma.
Unlike the situation described with canine and feline glaucoma, in developed countries enucleation is practically never carried out for uncomplicated primary open-angle glaucoma. It is, however, sometimes performed for the secondary types of glaucoma, particularly in cases of tumors, vascular disease, uveitis, and trauma.
THE CANINE GLAUCOMAS
Goniodysgenesis associated glaucoma (primary glaucoma, acute primary angle-closure glaucoma, primary open-angle closed-cleft glaucoma) (Figs 13.3, 13.4)
• The defining features of goniodysgenesis (pectinate ligament dysplasia, mesodermal dysgenesis)
▪ This is a familial, breed-related, congenital abnormality in the structures of the irido-corneal angle (ICA) and aqueous outflow apparatus
– Commonly affected breeds include the Bassett Hound, English and Welsh Springer spaniel, Flat-coated retriever, Great Dane, Samoyed, Sheba Inu and Siberian Husky
▪ Goniodysgenesis is characterized, morphologically, by the following features:
– A solid iris-like sheet of uveal stroma extending from the iris base to the arborized termination of Descemet’s membrane (pectinate ligament dysplasia)
– The abnormal sheets of uveal tissue are intermittent, and width of the irido-corneal angle and opening of the ciliary cleft can vary considerably throughout its circumference
○ The extent of goniodysgenesis is therefore best assessed by clinical observation of both the width of the opening of the ciliary cleft and the appearance of the pectinate ligament, using a special lens placed on the cornea (gonioscopy)
○ The glaucomatous eye cannot be used to observe the extent of goniodysgenesis because the cornea becomes cloudy with edema and the irido-corneal angle is narrowed by elevated IOP. The unaffected contralateral eye is used to evaluate the extent of affected angle
○ Severity or grade of goniodysgenesis appears to be heritable
○ The greater the extent of circumferential involvement of the drainage angle, the greater the chances of developing glaucoma
▪ Severe goniodysgenesis is clearly a risk factor in the development of glaucoma but most dogs affected by goniodysgenesis do not develop glaucoma
– Although gonioscopy may reveal goniodysgenesis or pectinate ligament dysplasia (PLD) in a significant number of dogs within certain breeds, only some of these animals will actually go on to develop glaucoma. The pectinate ligament is clearly not the most important source of resistance to aqueous outflow
– However, goniodysgenesis may signal the existence of other developmental abnormalities within the structures of the ciliary cleft or may have implications in the way the iris is positioned during miosis and mydriasis perhaps predisposing to pigment dispersion
– The limitation of gonioscopy in our clinical evaluation of the canine aqueous outflow pathway has been highlighted by histopathological identification of goniodysgenesis and ciliary cleft collapse in Norwegian elkhounds, a breed previously classified as affected by a chronic, slowly progressive, ‘open-angle’ glaucoma
▪ A dog with goniodysgenesis-related glaucoma in one eye is highly likely to develop glaucoma in the second eye
– For this reason there is a lot of interest in ‘prophylactic’ treatment which might delay or prevent glaucoma development in the second eye
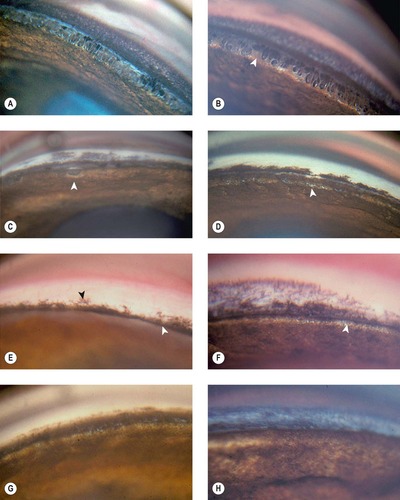 |
| Figure 13.3 Gonioscopy of the canine irido-corneal angle, normotensive eye, in goniodysgenesis. The following examples represent the ‘normotensive’ eye in cases presented with unilateral glaucoma. The glaucomatous eye was histopathologically diagnosed as goniodysgenesis. (A) Cocker Spaniel, 6 years old: the pectinate ligaments have a thick iris base. (B) Cocker Spaniel, 6 years old: the base of the pectinate ligaments is wide (arrow). This has been termed ‘truncated’ pectinate ligaments. (C) Cocker Spaniel, 9 years old: the sheet of pigmented tissue extends over most of the ciliary cleft. The arrow points to what is termed a ‘flow hole’ in this extensive sheet. (D) Cocker Spaniel: 6 years old: the fine white areas (arrow) represent small open areas in the heavily pigmented tissue. (E) Australian Cattle Dog, 4 years old: only the superficial pigmented band (black arrow) can be seen. The iris is ‘bowed’ forward occluding the view of the irido-corneal angle (white arrow). (F) Basset Hound, 4 years old: no normal pectinate ligaments could be visualized. The white band (arrow) may represent the ciliary cleft. (G) Dalmatian, 7 years old: the pigmented band covers the entire angle recess. (H) Bullmastiff, 2 years old: no ciliary cleft was visible. The bluish color may represent limbal corneal edema. |
 |
| Figure 13.4 Goniodysgenesis, pathology. (A) Gross photograph showing a canine irido-corneal angle with goniodysgenesis from a normotensive globe. (B) Photomicrograph of a normotensive eye with goniodysgenesis showing a solid membrane of iris-like tissue spanning the angle (arrowhead). The ciliary cleft and the corneoscleral trabecular meshwork (arrow) are normal in this normotensive eye. (C) Photomicrograph of goniodysgenesis sectioned through a small opening (arrow). (D) Photomicrograph of a canine angle with goniodysgenesis and glaucoma. The iris-like membrane is in front of a collapsed ciliary cleft (*) and the corneoscleral trabecular meshwork is not apparent. The terminus of Descemet’s membrane showing characteristic thickening and branching. |
• From ‘at risk’ to acute glaucoma – pathogenic mechanisms in dogs with goniodysgenesis
▪ In dogs with goniodysgenesis, the progression to an acute glaucomatous crisis characteristically does not occur until middle age or later in life
– There is a suspicion that in many affected dogs, transient episodes of IOP elevation, with subsequent spontaneous resolution, precede confirmed glaucomatous crises. There are competing hypotheses which attempt to explain the increased intraocular pressure
– Elucidating the mechanisms that precipitate acute IOP elevations in dogs with goniodysgenesis will greatly enhance our ability to treat, and hopefully prevent or delay the onset of, glaucoma in ‘at-risk’ eyes
▪ Severe goniodysgenesis, narrow ICA, relatively anterior lens position, thick lens, and shallow anterior chamber, may all be considered as anatomic risk factors, i.e. markers indicating predisposition to glaucoma
– Similar anatomic risk factors may predispose human and canine subjects to primary angle closure glaucoma. However, only a small proportion of individuals with these characteristic risk factors go on to develop glaucoma
▪ Age-related changes in morphologic features such as lens thickness and narrowing of the irido-corneal angle may, at least in part, contribute to angle closure and the development of glaucoma in middle-aged and older dogs
▪ Acute pupil block is a dynamic process that has also been put forward as a factor contributing to acute glaucomatous crises. It has been proposed that a very small segment of the iris, right at the pupil margin, is held in apposition against the anterior lens capsule by a complex dynamic mechanism that involves ‘iris stretch’ and combined ‘blocking forces’ generated by both the sphincter and dilator muscles.
– A role for ‘iris touch’, i.e. iris-lens contact, in the pathogenesis of IOP elevation in dogs with goniodysgenesis-related glaucoma is supported by the finding of pigment dispersion in affected eyes
▪ Significant uveal inflammation was also identified in canine eyes with acute glaucoma. Whether uveitis plays a role in precipitating episodes of glaucoma due to iris thickening or miosis, or whether inflammation is a secondary phenomenon in dogs with primary glaucoma remains unclear
– Resistance to the flow of aqueous caused, in some way by the abnormal uveal membranes or by matrix deposited by the membrane
– Obstruction of aqueous drainage at the level of the pupil associated with contact between the papillary margin of the iris and the lens
– Degeneration of the filtration apparatus secondary to entrapment of released melanin
– Any combination of mechanisms
▪ Histopathological studies are associated with important limitations, including the tendency to examine only globes obtained at a late stage in the disease process
▪ Disease progression in canine goniodysgenesis-associated glaucoma begins with the sudden development of severe disease. Morphologically, the disease progresses in a stepwise manner, consistent with a rapidly progressive disease course following a dramatic initiating event
Sequential changes in the drainage angle following acute IOP elevation in goniodysgenesis-related glaucoma
The first 24 h (Fig. 13.5)
• Atrophy of the corneoscleral trabecular meshwork
▪ The identification of this change, so soon after the apparent onset of acute glaucoma, suggests that there is latent disease building up to the glaucomatous crisis
• Incomplete collapse of the ciliary cleft
• Disruption of the posterior pigmented epithelium of the iris near the pupillary margin
• Free pigment granules within the anterior chamber and in the angle and trabecular meshwork
• Neutrophilic inflammatory cell infiltrate in the anterior chamber, irido-corneal angle, ciliary cleft and the limbal sclera
• Nuclear enlargement and increased mitotic activity involving stromal fibroblasts and endothelial cells at the limbus
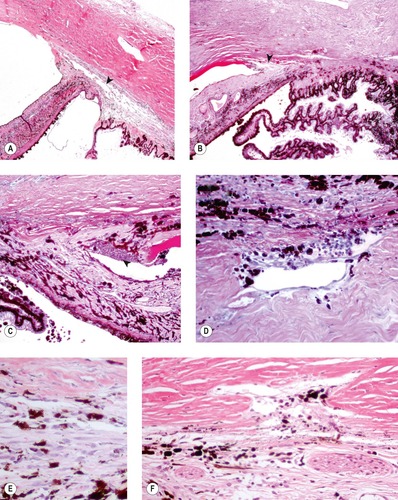 |
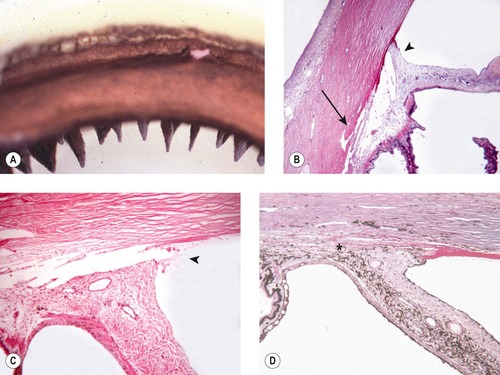
| Figure 13.5 Goniodysgenesis, pathology of acute disease. (A,B) Low magnification photomicrographs showing the canine irido-corneal angle from a dog with clinical signs of glaucoma for only 2 days. The ciliary cleft is still apparent, but not the corneoscleral trabecular meshwork (arrows). (C) This irido-corneal angle is from a dog with goniodysgenesis within 2 days of the onset of clinical disease. It shows a neutrophilic infiltrate (arrow). (D) The corneoscleral trabecular meshwork, from a dog with clinical glaucoma less than 2 days, shows pigment dispersion and a neutrophil infiltrate. (E,F) Atrophic corneoscleral trabecular meshwork from dogs with acute glaucoma. The structure is severely atrophied and contains excess pigment cells. |
1–5 days (Fig. 13.6)
• Complete closure of the ciliary cleft with indistinguishable corneoscleral trabecular meshwork
• Dispersion of pigment remains a prominent feature within the anterior chamber and filtration angle
• Loss of the posterior pigmented epithelium from the pupillary margin of the iris
• Neutrophilic inflammatory cell infiltrate is rarely seen
• Evidence of cellular proliferation remains apparent within the limbal tissues
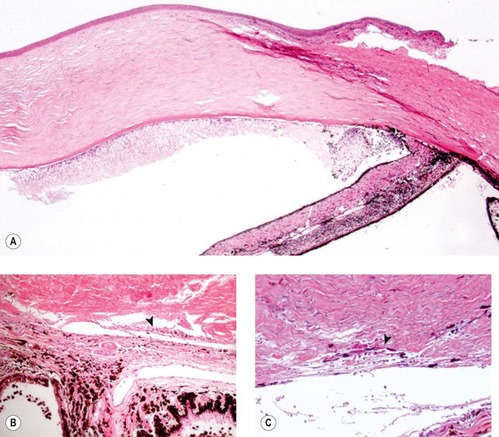 |
| Figure 13.6 Goniodysgenesis and pigment dispersion in acute glaucoma. (A) Low magnification photomicrograph from a dog with a 4-day history of glaucoma and goniodysgenesis showing large amounts of free pigment in the dependent aspect of the anterior chamber and complete collapse of the ciliary cleft. (B,C) Higher magnification photomicrographs showing what remains of the atrophied corneoscleral trabecular meshwork. Notice the pigment in the atrophied structure (arrows). |
Chronic changes (Fig. 13.7)
• Complete collapse of the ciliary cleft with indistinguishable corneoscleral trabecular meshwork
• No neutrophilic inflammation
• Pigment dispersion remains, but rarely at a significant level
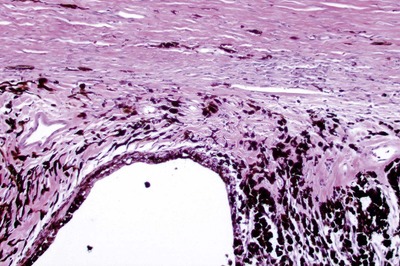 |
| Figure 13.7 Goniodysgenesis, chronic disease. Photomicrograph, from a dog with glaucoma for 1 week or longer, showing the completely collapsed and atrophied ciliary cleft and corneoscleral trabecular meshwork. |
Sequential changes in the retina following acute IOP elevation in goniodysgenesis-related glaucoma
The first 24 h (Figs 13.8, 13.9)
• Radiating sectors of edematous retina apparent grossly
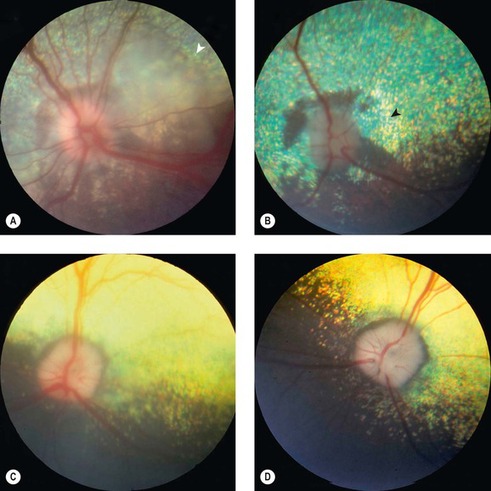 |
| Figure 13.8 Retinal disease in acute goniodysgenesis glaucoma, fundus. (A) Samoyed, 4 years old: the optic disc is swollen and retinal vessels are dilated. The peripapillary retina is edematous and has a large bullous detachment (arrow). (B) The same eye as in (A), 12 days following treatment, is now a normotensive eye. The optic disc is pale. Peripapillary tapetal pigmentation and hyperreflectivity (arrow) are present. The eye was visual. (C) Cocker Spaniel, 8 years old: the peripapillary retina is edematous. The optic nerve changes are discussed in Figure 13.12. (D) This is the same eye as (C), 4 weeks later. The peripapillary retina is atrophic, resulting in tapetal hyperreflectivity. The eye was visual. |
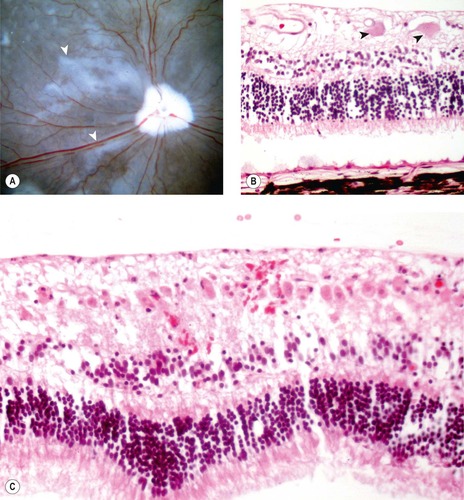 |
| Figure 13.9 Retinal disease in acute goniodysgenesis glaucoma, pathology. (A) Gross photograph of a canine globe posterior segment from a dog with clinical glaucoma for 3 days before enucleation. The globe, which was fixed in glutaraldehyde and opened so that the fundus is visible, showing radiating spokes of translucent retina (arrows). (B) Photomicrograph, of the retina from a dog with glaucoma for 1 day, showing edema and hypereosinophilic necrotic ganglion cells (arrows). In glaucoma, necrotic ganglion cells can reliably be found histologically only in dogs that have had enucleation within 2 days after the onset of clinical disease. (C) Photomicrograph, of the retina from a dog that had optic nerve trauma and enucleation 2 days later, showing extensive ganglion cell necrosis, which suggests that a wave of ganglion cell necrosis occurs before the clinical onset of glaucoma in dogs. |
• Hypereosinophilic, necrotic retinal ganglion cell profiles seen
▪ This change is seen rarely at later stages, in association with more chronic disease, suggesting that retinal degeneration can occur as a cyclic or ongoing process
• Outer retinal edema but no cellular necrosis or apoptosis
• Müller cell expression of glial fibrillary acidic protein (GFAP) is little changed from background
• Neutrophilic inflammatory cell infiltrate in the retinal tissue
1–5 days (Fig. 13.10)
• Radiating zones of retinal edema and degeneration still apparent grossly
• Extensive, segmental, full-thickness retinal cell apoptosis. The pattern of regionally variable severity correlates with the radiating pattern of edema and degeneration seen grossly
• Segmental full-thickness retinal degeneration and necrosis is characteristic in dogs with acute goniodysgenesis-related glaucoma, but is not a typical feature associated with glaucoma in any other species
• Marked upregulation in the expression of GFAP by Müller cells and astrocytes
• Degenerative changes often appear more advanced within the dependant, non-tapetal, retina (a phenomenon referred to as ‘tapetal sparing’)
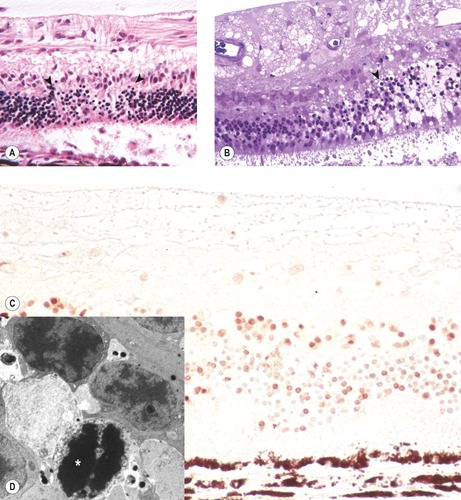 |
| Figure 13.10 Retinal pathology in acute goniodysgenesis glaucoma. (A) Photomicrograph, of the retina from a dog with a 4-day history of glaucoma, showing segmental apoptosis between the arrows. (B) Plastic section, of the retina from a dog with a 4-day history of glaucoma, showing segmental severe edema and apoptosis (arrow) Toluidine blue stain. (C) Photomicrograph showing the retina from a dog with 5-day glaucoma. Numerous apoptotic cells are demonstrated with the TUNEL method brown staining nuclei (D) Electron micrograph from the same dog as (B) showing remnants of the nucleus of a photoreceptor cell undergoing apoptosis (*). |
Chronic changes (Fig. 13.11)
• Full thickness retinal atrophy with elevated expression of GFAP
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


