Numerous references to plagues that may have been caused by Y. pestis appear in ancient literature, including the Old Testament. The first documented plague epidemic occurred in Athens in 430 BC; it resulted in the death of one third of the population and precipitated the downfall of classical Greece. Outbreaks between AD 541 and 750 comprise the Justinian pandemic, beginning in Ethiopia and spreading from Egypt through the Middle East and into Mediterranean Europe. Epidemics occurred between AD 558 and 654 in 8- to 12-year cycles in North Africa, Europe, and Asia, with mortality rates approaching 50%. The so-called second pandemic (1330-1346) originated in central Asia; Mongolian plague victims were catapulted over the city walls of Caffa in 1346. Plague spread westward along trade routes, mediated by sales of furs from animals dead of plague, and entered Europe by way of Messina, Genoa, and Marseilles in 1347. It was during these epidemics that plague came to be known as the Black Death, killing as many as 28 million (40% of the population). The disease recurred in regular cycles until the early 1700s. Cases arrived in southern France from Syria and Lebanon in early 1720, and the ensuing epidemic killed 50,000 people in Marseilles. Examination of the graves from this period provided the first evidence that bronze pins were driven into the toes to verify death. The third pandemic began in 1855 in China, progressing rapidly to Hong Kong and beyond, including Hawaii and then North America.
Alexandre Yersin, a protègè of Louis Pasteur sent to Hong Kong to study the plague, and the Japanese scientist Shibasaburo Kitasato independently isolated the plague bacillus within a few days of each other, and the organism was originally named Pasteurella pestis in honor of Pasteur. Yersin described a connection between rats and plague, and Paul Louis Simon discovered the role of the rat flea in transmission. Enzootic foci of plague are found today on every continent except Australia, and the World Health Organization now classifies it as a reemerging infectious disease.
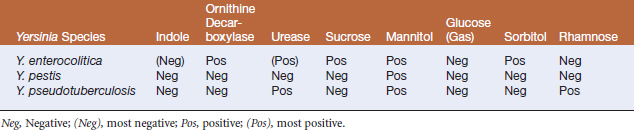
Molecular analysis has revealed that Y. pestis is a subspecies of Y. pseudotuberculosis, although the name has been retained. Yersinia pestis is thought to have emerged as a separate clone within Y. pseudotuberculosis shortly before the first pandemics. Biotypes of Y. pestis are distinguished by their differential ability to reduce nitrate and to ferment glycerol and melibiose. Biovar Antiqua probably spread from central Asia to central Africa to cause the first pandemic. Biovar Medievalis (similar genetically, but unable to reduce nitrate) later emerged (also in central Asia), spreading to Crimea and causing the second pandemic. Later still, biovar Orientalis emerged to cause the third pandemic.
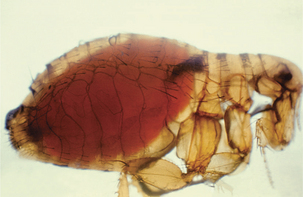
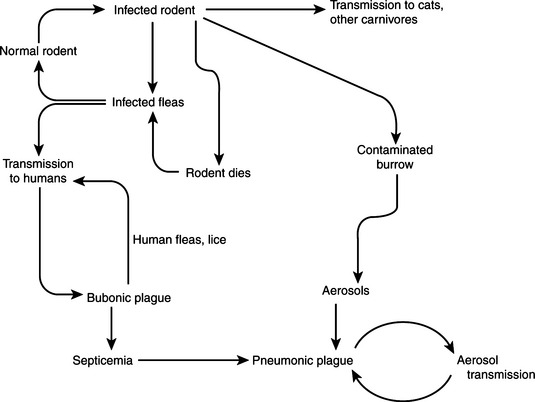
Fleas ingest Y. pestis in blood meals from septicemic animals, and in most cases the organism proliferates in the normally sterile flea midgut (Figure 17-2). The proventriculus is a spine-lined organ that lies between the esophagus and midgut. In addition to mechanically disrupting blood cells, it serves as a valve, permitting entry of blood to the midgut while precluding escape of ingested blood. Yersinia pestis attaches in aggregates to the proventricular surface and, with continued multiplication, causes blockage and, in effect, starvation of the flea (Figure 17-3). In response, fleas attempt to feed more often. Blood enters the esophagus, becomes infected with Y. pestis, and is pumped back into the bite wound. Fleas do not block at higher temperatures, and dogma is that bubonic plague epidemics end with the onset of warmer temperatures. The temperature-sensitive activity of plasminogen activator in allowing or prohibiting flea blockage may be related to this phenomenon.
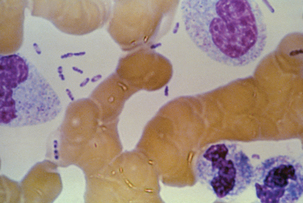
Sylvatic plague is generally transmitted by the bite of an arthropod, and symptoms appear after an incubation period of 2 to 6 days. It proliferatesto some degree at the site of the bite. The organism is carried to regional lymph nodes, where proliferation occurs with enthusiasm. Inflammation, necrosis, and swelling in the lymph node lead to formation of the bubo, a classic gross lesion from which bubonic plague derives its name. Invasion may be contained by the antibacterial effectsof lymph node residence, but the organism isalso likely to escape, eventually entering circulation and causing massive septicemia (Figure 17-4). Additional foci of infection develop in liver, spleen, and lungs, the last often resulting in airborne spread and the development of primary pneumonic cases of urban plague. There is a strong correlation between substantive bacteremia and subsequent mortality, and in any case, untreated pneumonic plague rarely has a clinical course longer than3 days. Primary pneumonic plague, without the bubonic form as a precursor, is most common among plague cases in the United States today.
In addition to rodents, a number of mammalian species have been found to be naturally infected with Y. pestis, including lagomorphs, felids, canids, mustelids, and some ungulates.
Pathologic changes in feline plague are akin to those in humans. After introduction by fleabite or contact with infected material, Y. pestis multiplies locally and is transferred via lymphatics to regional lymph nodes. Nodal necrosis and suppuration, with hemorrhage, edema, fibrin, and acute necrotizing inflammation, are common. Large numbers of Y. pestis are in evidence in blood and lymphatic vessels associated with lymph nodes. Dissemination beyond regional lymph nodes to circulation eventually involves spleen, liver, heart, lungs, and other organs. Pneumonic plague may result, or may be the primary clinical presentation, through inhalation of infectious aerosols. Pulmonary lesions in cats include diffuse interstitial pneumonia and devastation of lung architecture by coalescing necrotic areas of necrosis. Lung abscesses are uncommon.
The clinical presentation of feline plague is rapidly progressing febrile illness with an incubation period of 1 to 4 days. Cats with primary septicemia are lethargic and anorexic, with signs of sepsis including vomiting, diarrhea, weak pulse and tachycardia, cold extremities, and disseminated intravascular coagulopathy (DIC). The bubonic form is most common, and one or more enlarged, abscessed, or draining lymph nodes are painful on palpation. Some infected individuals do not develop classic signs, so plague should be part of the differential diagnosis in cats with a suggestive history and a systemic infectious process.
Most cats exposed experimentally to Y. pestis (by subcutaneous inoculation or feeding of mice dead of plague) develop infection. In affected cats, rectal temperature peaked at 40.5° to 41.1° C on the third days after inoculation. Nearly two thirds of clinically ill cats developed enlarged cervical or cranial lymph nodes in the head and neck region within 4 to 6 days, and half died. Oral exposure is more likely to result in bubo formation. In natural cases, lymphadenopathy is most likely to be unilateral and submandibular. Oral and lingual ulcers or abscesses have also been reported.
Dogs are less likely to develop severe clinical illness following natural or experimental exposure. Fever, lethargy, submandibular lymphadenitis, suppurative intermandibular lymphadenitis, oral lesions, and cough have been documented in naturally infected dogs. Antibodies suggestive of plague exposure have been found unexpectedly in dogs in areas with low elevation and high average temperatures. This, and their relative resistance to clinical disease, suggests that dogs may be useful as sentinels.
Cattle, horses, sheep, and pigs are apparently not susceptible to clinical manifestations of plague. Goats and camels, on the other hand, are susceptible and human plague has been associated with ingestion of Y. pestis–infected camel meat in the Middle East. Disease has also been reportedin a llama, mule deer, and antelope, and effects in mountain lions and bobcats may be similar to those in domestic cats. Foxes, raccoons, skunks, bears, and coyotes are apparently resistant, and the last two species are often used as natural sentinels.
Yersinia pestis is widely acknowledged to have been weaponized in modern times for use in biological warfare and bioterrorism. The United States and the former Soviet Union investigated the use of plague as a biological weapon during the cold war. A plague-based weapon delivered by aerosol would likely cause signs consistent with severe community-acquired pneumonia, and confusion with more common respiratory illnesses (e.g., legionnaires’ disease) may delay recognition. True Y. pestis pulmonary infections may thus progress rapidly to septic shock and death without early treatment. The organism is a Category A Critical Biological Agent.