Chapter 11 The Genus Mycobacterium
Mycobacteria cover the range from saprophytes to opportunists to obligate pathogens, and have close relatives in other genera of pathogenic bacteria (Table 11-1). All members of the genus are aerobic, acid-fast, non–spore-forming, nonmotile gram-positive rods. They often stain irregularly and appear somewhat beaded. They are catalase positive. Many produce pigments, which are a means of classification and differentiation; scotochromogenic organisms produce pigments whether incubated in light or dark, whereas in photochromogenic organisms, light is required for pigment production (Figure 11-1). Most mycobacteria have relatively simple growth requirements. Mycobacterium tuberculosis, for example, can be cultivated in a synthetic liquid medium with only trace metals, asparagine, and glycerol. Rapidly growing mycobacteria produce colonies on solid media in less than 7 days, and slow growers require 7 to 14 days (or longer, if growth must be initiated from low-titer inocula), and, in some cases, as long as several months, to exhibit recognizable colonial growth. To put this in perspective, the generation time of Escherichia coli is usually accepted to be about 15 to 20 minutes, whereas that of Corynebacterium diphtheriae is closer to 60 minutes. Mycobacterium tuberculosis, on the other hand, completes a single round of cell division in about 300 minutes; Mycobacterium avium ssp. paratuberculosis is perhaps the most slow-growing of all the mycobacteria, in that cultures for diagnosis of Johne’s disease are not discarded as negative until they have incubated for greater than or equal to 5 months.
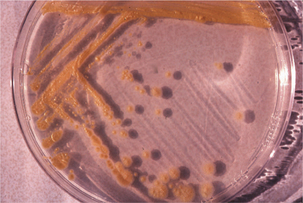
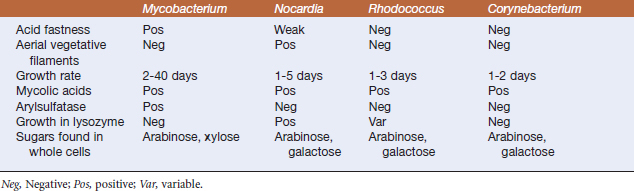
FIGURE 11-1 Mycobacterium marinum, cultivated on Middlebrook’s 7H10 agar.
(Courtesy J. Glenn Songer.)
Acid fastness refers to the ability of mycobacterial cells to bind phenol-based dyes (for example, carbol fuchsin in 5% phenol), typically when heated while staining; the dye is retained whenthe smear is subsequently treated with acidified alcohol. Whereas characteristic of mycobacteria, acid-fastness occurs in other genera of bacteria and even in certain life stages of some eukaryotes.
Complex lipids in mycobacterial cell walls include the mycolic acids, which are also found in related genera (Figure 11-2; see Table 11-1); acid fastness relates to the presence of peptidoglycan and glycolipids. This cell wall composition is also responsible for resistance of mycobacteria to drying, extremes of pH, and other environmental stresses. The complex, lipid-rich cell wall alsoprotects the organism in the phagolysosome, and probably plays a major part in mycobacterial survival in macrophages. Furthermore, components of the cell wall are immunostimulatory, and are the basis for adjuvants, including Freund’s complete and N-acetyl-muramyl-L-alanyl-D isoglutamine, or muramyl dipeptide (MDP).
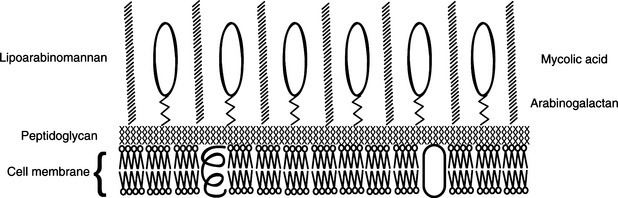
FIGURE 11-2 Schematic representation of mycobacterial cell wall composition.
(Courtesy Ashley E. Harmon.)
Some mycobacteria are major pathogens of domestic animals, whereas others are encountered only occasionally (Table 11-2).
TABLE 11-2 Mycobacteria of Veterinary Significance
| Mycobacterium Species | Disease | Cultural Features |
|---|---|---|
| M. tuberculosis | Human tuberculosis | Rough, raised, thick; nodular or wrinkled surfaces; white to buff, very light yellow |
| M. africanum | Human tuberculosis (Africa); tuberculosis in primates, hoofed animals, dogs, swine, other species | |
| M. bovis | Tuberculosis in cattle, other ruminants; humans, dogs, cats, swine, rabbits, subhuman primates | Small, rounded, white, irregular edges, granular surface |
| M. bovis ssp. caprae | Caprine tuberculosis | |
| M. microti | Vole tuberculosis | |
| M. avium ssp. avium | Avian tuberculosis, swine mycobacteriosis (lymphadenitis); granulomatous disease (usually lymphadenitis) in cats, dogs, ruminants; disease in cold-blooded species; lymphadenitis, progressive disease in humans; rare intestinal lesions in horses, pigs, others | Transparent to opaque, smooth, “asteroid” margins; may become yellow with age |
| M. intracellulare | ||
| M. avium ssp. paratuberculosis | Ruminant paratuberculosis (Johne’s disease); associated with human Crohn’s disease | Colorless to white, translucent |
| M. genavense | Tuberculosis in psittacine birds | Smooth, thin to transparent, nonchromogenic |
| M. scrofulaceum | Porcine lymphadenitis | Raised, rounded, pale orange |
| M. porcinum | Porcine lymphadenitis | Rough, buff-to-whitish colonies |
| M. simiae | Tuberculosis-like disease in monkeys, humans | Smooth, usually photochromogenic |
| M. ulcerans | Feline ulcerative/nodular skin lesions | |
| M. xenopi | Feline ulcerative/nodular skin lesions; porcine cervical, mesenteric lymphadenitis | |
| M. kansasii | Human lymphadenitis, lung disease; lymphadenitis in cattle, swine | Smooth to rough, colorless to buff; photochromogenic (bright yellow pigment) |
| M. marinum | Granulomatous disease in fish, other species (marine and freshwater); human granulomatous disease | Smooth to rough colonies; appear in approximately 7 days incubation at 30° C, more quickly at 25° C; brilliant yellow |
| M. fortuitum | Bovine granulomatous mastitis; piscine granulomatous disease; feline ulcerative pyogranulomatous skin disease; canine granulomatous lung and skin disease; porcine granulomatous joint and lung disease | Small, rough, buff colored, waxy, convex; entire edges; peculiar odors |
| M. peregrinum | ||
| M. chelonae | Piscine granulomatous disease; tuberculosis-like lung lesions in turtles; bovine granulomatous lymphadenitis; feline, porcine abscesses, ulcerative lesions; lymph node abscesses, disseminated disease in monkeys | Small, rough, buff colored, waxy, convex; entire edges; peculiar odors |
| M. abscessus | ||
| M. farcinogenes | Bovine farcy (Africa) | |
| M. senegalense | Bovine farcy (Africa) | |
| M. vaccae | Bovine skin disease | |
| M. smegmatis | Bovine granulomatous mastitis; feline ulcerative skin disease | Rough, wrinkled or coarsely folded, butyrous, glistening; nonpigmented or creamy white |
| M. phlei | Feline ulcerative skin disease (rare) | |
| M. leprae | Human leprosy; granulomatous disease in armadillos, other species | |
| M. lepraemurium | Possible cause of feline, murine leprosy |
MYCOBACTERIUM TUBERCULOSIS COMPLEX
Infection by M. tuberculosis is primarily a problem in humans and subhuman primates, but dogs, canaries, psittacine birds, swine, and many other species are susceptible to human tuberculosis. Feline cutaneous tuberculosis is associated with infection by M. tuberculosis or M. bovis and presents as multiple exudative ulcers and abscesses, in the form of pyogranulomatous dermatitis with caseous necrosis. Mycobacterium tuberculosis should be part of the differential diagnosis in any granulomatous disease of warm-blooded animals.
Pathogenesis
Regardless of the route of infection, the organism is phagocytosed by macrophages, probably following complement-mediated opsonization. Defense of the lungs centers, of course, onthe work of pulmonary alveolar macrophages;survival and multiplication of M. tuberculosis in phagocytes is the key factor in development of disease. Phagosome-lysosome fusion occurs, but the organisms either escape to the cytoplasm or simply multiply within the phagolysosome, eventually causing it to burst. Part of the success of M. tuberculosis in this endeavor lies in its abilityto prevent acidification of the phagosome, which decreases the killing capacity of phagocytes. Mycobacteria in general are relatively resistantto the bactericidal mechanisms of professional phagocytes, with resistance to reactive oxygen inter-mediates associated with catalase, peroxidase, and alkyl hydroperoxidase reductase production; the last also mediates resistance to reactive nitrogen intermediates. Mycobacterial sulfolipids may inhibit phagosome:lysosome fusion and potentiate the cord factor–induced inhibition of oxidative phosphorylation in mitochondria. Phenolic glycolipids in the cell wall scavenge and detoxify oxygen radicals. Furthermore, M. tuberculosis produces compounds that may interfere with T-cell activation. Lipoarabinomannan, a mycobacterial cell wall glycolipid, suppresses T-cell proliferation, blocks transcriptional activation of interferon (IFN)-γ–inducible genes in macrophages cell lines, and might prevent macrophage activation by IFN-γ. A fibronectin-binding protein may be involved in fibronectin depletion, making it unavailable for binding and stimulation of T cells and interfering with the activated macrophage response.
If the immune response is delayed or nonex-istent, viable M. tuberculosis may reach regional lymph nodes and even pass farther by way of lymphatics and the bloodstream, to distant tissues, nearly always within macrophages. Most bacteria, however, are contained locally, in a specific typeof granuloma called a tubercle (Figure 11-3); layers of T cells, neutrophils, macrophages, multinucleated giant cells, and a thick fibrincoat form around growing foci of necrosisand sometimes wall off the lesion. Calcified tubercles appear as lesions in chest radiographs. The walled-off lesions may contain viable bacteria, leaving open the possibility of reactivation tuberculosis.
FIGURE 11-3 Hematoxylin and eosin–stainedsection of a tubercle in a case of bovine tuberculosis.
(Courtesy J. Glenn Songer.)
Laboratory Diagnosis of Tuberculosis
Diagnosis is based in part on microscopic exami-nation of acid-fast–stained sputum smears. Fluo-rochrome stains (auramine O-acridine orange) are also useful, and evidence suggests that they are more sensitive than traditional acid-fast stains. Bacteriologic culture of appropriate specimens is imperative, both to confirm the etiology and allow for sensitivity testing. Isolation is facilitated by the resistance of the organism to disinfectants and extremes of pH. Steps in the procedure may include treatment with N-acetyl L-cysteine (to liquefy sputum samples), exposure to high pH, or disinfection with quaternary ammonium compounds. Culture is often on inspissated egg-based media (such as Lowenstein-Jensen), agar-based egg media (such as Herrold’s medium), or non-egg media (such as Middlebrook’s 7H10), prepared as slants to allow humidity control. Mature colonies become apparent after 14 to 21 days’ incubation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree