Chapter 32 The Genera Treponema and Borrelia
THE GENUS TREPONEMA
Treponema spp. are motile, helical rods with tight, regular to irregular spirals (Figure 32-1). Many species are normal flora in the oral cavities, genital tract, or rumen of animals.
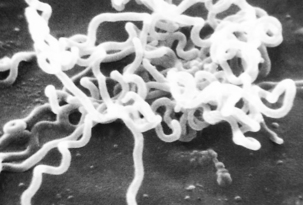
FIGURE 32-1 Electron photomicrograph of Treponema pallidum on cottontail rabbit epithelial cells.
(Courtesy Public Health Image Library, PHIL #1971, Centers for Disease Control and Prevention, Atlanta, 1980, David Cox.)
DISEASE AND PATHOGENESIS
The incidence of human syphilis is increasing, as is often the case with diseases having such a strong sociologic component. Primary syphilis is evidenced by the development of a circumscribed lesion, called a chancre, at the inoculation site (Figure 32-2). It is initially maculopapular, but eventually develops an inflamed, necrotic, moist center. Exudate from the chancre teems with spirochetes, and this is the most infectious stage of the disease for person-to-person transmission. The chancre may be in a location that is not readily visible and may thus go unnoticed. Treatment at this stage results in a nearly 100% cure rate, but the lesion disappears without treatment in 10 to 14 days.
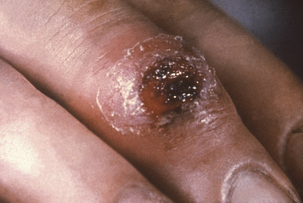
FIGURE 32-2 Extragenital syphilitic chancre on the right middle finger.
(Courtesy Public Health Image Library, PHIL #4146, Centers for Disease Control and Prevention, Atlanta, date unknown.)
Elucidation of virulence mechanisms of T. pallidum has been limited by failure of all attempts to cultivate it in vitro. Furthermore, there is no suitable in vivo model of human disease. Treponema pallidum can be maintained by serial passage in rabbit testicles, a process in which laboratory technicians have approximately the same risk of occupational exposure to the organism as street-based sex workers. However, symptoms (in the rabbits) are limited to rather severe orchitis in steroid nontreated animals, and no visible symptoms at all if steroidal antiinflammatory drugs are used. Chancres resembling those in human syphilis are produced by intradermal inoculation of rabbit skin, and these resolve much as would be expected in humans. However, the infection does not become systemic. Information gleaned from study of natural cases and limited work with in vivo–grown organisms has revealed that T. pallidum adheres to and invades cell monolayers (Figure 32-3).
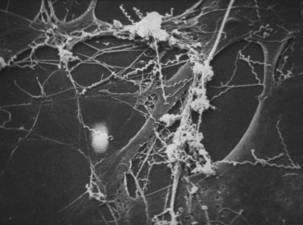
FIGURE 32-3 Electron photomicrograph of Treponema pallidum on cottontail rabbit epithelial cells.
(Courtesy Public Health Image Library, PHIL #1975, Centers for Disease Control and Prevention, Atlanta, 1980, David Cox.)
Treponema pallidum has a surprising lack of outer-membrane proteins, and the treponemal equivalent of porin activity (to allow diffusion of nutrients through the outer membrane) remains undiscovered. There is likely some sort of active transport mechanism, because T. pallidum is susceptible to β-lactam antibiotics, and these are not thought to diffuse through membranes.
DIAGNOSIS
Treponema brennaborense and Hairy Footwart
Disease and Etiology.
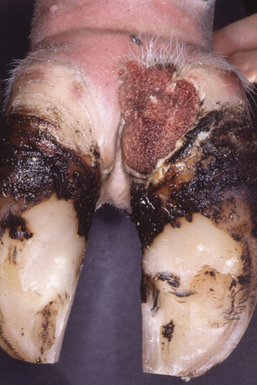
The incubation period of PDD is about 3 weeks. Its clinical presentation is episodic lameness of variable severity, as a result of acute or chronic ulceration of the skin on the bulbs of the heel or the interdigital cleft and often just above the perioplic horn of the heels. In intense cases, cows often walk on their toes and hooves are clubbed. Lesions are most commonly associated with plantar or palmar skin adjacent to the interdigital space (Figure 32-4). Both medial and lateral digits of individual limbs are involved in most animals.
A novel treponeme isolated from typical lesions was determined, based on chemotaxonomy, protein profiling, and analysis of 16S rDNA sequences, to be a new species, and was named T. brennaborense. It is small and highly motile, with two periplasmic flagella arranged in a 1-2-1 pattern (Figure 32-5). It has α-glucosidase and N-acetyl-β-glucosaminidase activity, and growth is inhibited by rabbit serum. Comparative 16S rDNA sequence analysis of multiple isolates revealed three phylotypes clustered with the saprophytic human oral (Treponema denticola and Treponema vincentii) or genital (Treponema phagedenis) treponemes. Treponema brennaborense is probably most closely related to Treponema maltophilum, from human periodontitis. Differential distribution in lesions suggests that development of deep lesions may correlate with the presence of a particular phylotype or combination of phylotypes. Immunohistochemical examination of lesion biopsies reveals that organisms from many countries are antigenically related.
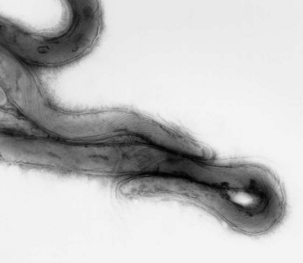
FIGURE 32-5 Electron photomicrograph of Treponema brennaborense, showing axial fibrils.
(Courtesy Richard L. Walker.)
Pathogenesis of T. brennaborense infections has not been explored.
Diagnosis
Diagnosis of PDD is based on observation oftypical clinical signs and lesions and detectionof morphologically compatible spirochetes in biopsies stained by silver impregnation or other methods (Figure 32-6).
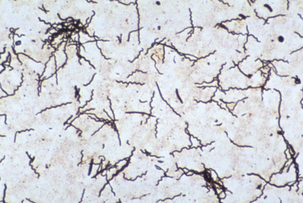
FIGURE 32-6 Treponema brennaborense in a stained smear from a papillomatous digital dermatitis (PDD) lesion.
(Courtesy Richard L. Walker.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree