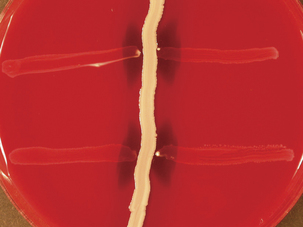
Streptococcus pyogenes (group A) is a paradigmatic extracellular gram-positive pathogen causing pharyngitis, impetigo, rheumatic fever, and acute glomerulonephritis in humans; it is a rare cause of bovine mastitis, often contracted from carriers among the milkers. Invasive streptococcal diseases (the so-called flesh-eating bacteria) and rheumatic fever in humans have become prominent over the decade of the 1990s.
Humans are the major reservoir hosts for group A streptococci. It is worth noting that although pediatricians frequently implicate companion animals as a source of infection for children, the evidence supports the opposite conclusion: pets are more likely to become culture positive by contact with infected children.
Streptococcal virulence is based in large part on antiphagocytic surface components, including the M protein. Its hypervariable N-terminal portion gives rise to different types, which may be determined by serologic methods or, more accurately, by emm typing, based upon the sequence of the hypervariable region of emm. The emm superfamily includes antiphagocytic molecules and immunoglobulin-binding proteins. Pyrogenic exotoxin A is one of nine superantigens that contribute to the pathogenesis of streptococcal toxic shock syndrome by stimulating cytokine production by T cells, with subsequent endothelial cell damage, hypotensive shock, and ischemia-based necrosis.
Enzymes that dissolve fibrin clots and other somatic and soluble toxic molecules also participate. Binding of plasma components may enable the organisms to evade host immune detection or to avoid the opsonophagocytic effects of complement. Factor H and fibrinogen binding by M protein are important virulence attributes, as is degradation of complement component C5a by the C5a peptidase. Mimicry of host molecules not only allows the organism to escape detection as foreign but may prompt the autoimmune responses associated with rheumatic fever; proteins in some strains are involved in immune-mediated acute glomerulonephritis, which sometimes follows streptococcal infection in humans. Production of capsular hyaluronic acid may be coregulated, with M protein and C5a peptidase, by virR. Loss of capsule is associated with reduction in virulence in the range of 100-fold.
Streptolysin O (SLO), produced by streptococci of groups A, C, and G, is a cholesterol-binding toxin with potent membrane-damaging effects. Individual SLO molecules associate with cholesterol in cell membranes and then oligomerize, inserting into the membrane and forming a hydrophilic channel. Toxicity is expressed against neutrophils, platelets, myocardial cells, and membrane-enclosed subcellular components.
Streptococcus agalactiae, the only member of group B, is best known as a cause of chronic bovine mastitis. In cattle, its nearly exclusive localization to the mammary gland facilitates eradication of the organism from affected herds. The organism also causes mastitis in sheep, goats, and camels, and has been associated with folliculitis in elephants, neonatal death, endocarditis, and vaginal and skin infections in dogs, and kidney and uterine infections in cats. Streptococcus agalactiae also causes rare infections in horses (genital tract) and monkeys.
In humans it is found in the vagina and in the oropharynx, and epidemiologic evidence suggests that this may be perpetuated by mixing and matching of mucous membranes. Early or late-onset meningeal sepsis in human neonates follows infection of the fetus in the birth canal, and S. agalactiae is in fact the most common cause of this type of human infection. Animal-to-human transmission probably does not occur, and substantial differences in phenotype suggest that bacteria from each host are distinct. Most isolates of S. agalactiae can be typed based on polysaccharide capsular antigens (types Ia, Ib Ic, II, and III).
Most study of virulence of S. agalactiae has focused upon human isolates and infections, and knowledge of the role of specific virulence factors in bovine mastitis is largely derivative. Human strains produce sufficient capsular polysaccharide to inhibit complement activation and C3 deposition, but bovine strains usually do not. Capsular polysaccharide is antiphagocytic, and type-specific antibody is protective in both the mouse model and in human infants. Anti–group B antibodies are opsonic in concert with complement.
Colonization of the mammary gland by S. agalactiae is facilitated by adhesion to gland sinus epithelium. Progressive inflammation and fibrosis result from multiplication on the teat epithelium and in duct sinuses. Isolation of the organism from supramammary lymph nodes early in the infection is evidence of transient invasiveness, but a strong neutrophil response clears these organisms rapidly. Contents of dead neutrophils accentuate the inflammatory process and formation of fibrin plugs in milk ducts may lead to loss of milk-producing capacity. The host response alone is not sufficient to clear the infection, so without treatment it often becomes chronic.
Strains of S. agalactiae augment the hemolytic activity of staphylococcal β-toxin via the action of the CAMP factor (Figure 5-1), a ceramide-binding protein. CAMP factor is lethal for laboratory rodents, and mutants lacking it have a 50-fold increased LD50. The question of CAMP factor toxicity for the mammary gland remains open. Neuraminidase and hemolysin may also play a role in development of mastitis.
Streptococcus canis (group G) comprises large-colony β-hemolytic organisms that produce β-galactosidase but not hyaluronidase (by which they are distinguished from Streptococcus dysgalactiae) and cause genital, skin, and wound infections in dogs. Dogs and cats can be genital or oral carriers of S. canis, but the main source of the organism, especially in dogs, is the anal mucosa. Infections are opportunistic and consist of abscesses, mastitis, prostatitis, lymphadenitis, pyometra, pyoderma, and “puppy strangles.” Streptococcus canis is commonly isolated from feline abscesses, metritis, mastitis, and kitten septicemia. Although Streptococcus zooepidemicus ssp. equi infection is more common, S. canis lymphadenitis has been reported in rats. Group G streptococci from humans and domestic animals are genetically distinct, but S. canis has nonetheless been associated with caretaker-associated bovine mastitis.
Streptococcal toxic shock, with or without necrotizing fasciitis, is apparently emerging as a disease problem in dogs. Organisms isolated from these cases are group G, and although most are S. canis, strains characteristic of those from human disease have also been found. The most common primary source of infection in toxic shock cases is the lung, with dogs experiencing acute or peracute suppurative bronchopneumonia. The case history sometimes includes failed attempts to treat with enrofloxacin; corticosteroids or nonsteroidal anti-inflammatory drugs also have been associated with infection. Septicemia and toxic shock are uniformly fatal, whereas most dogs with necrotizing fasciitis alone survive with radical treatment. Most isolates have the genetic machinery for production of SLO and M protein, but lack genes with homology to other virulence genes found in S. pyogenes.
Neonatal kittens are infected via the umbilicus, with the organisms originating from vaginal or oral colonization of the queen. Streptococcus canis is distributed hematogenously, and disseminated microabscesses result, with death following in the first week of life. This form of the disease occurs only in litters from young queens. Young cats can also be infected when maternal antibody titers decrease below effective levels, at 2 to 4 monthsof age. Organisms invading from the oral cavity cause purulent lymphadenitis involving the mandibular lymph nodes.
Little is known of the virulence factors of S. canis. Hyaluronidase and streptokinase are not involved, but large amounts of SLO and M protein are produced by low-passage clinical isolates. Antiserum against M protein is opsonic.
The taxonomy of S. dysgalactiae has changed substantially in recent years. The genetic similarity of the former Streptococcus equisimilis to S. dysgalactiae, as well as strains in groups G and L, led to their placement in S. dysgalactiae, with subspecies dysgalactiae and equisimilis. Strains identified as Streptococcus dysgalactiae ssp. equisimilis are mainly of group C, with some of group G (Figure 5-2); a not-insignificant number have group A and L antigens. Streptococcus dysgalactiae ssp. dysgalactiae has not been isolated from humans, despite the fact that it produces M-like proteins and other virulence attributes similar to those of S. pyogenes. Subspecies equisimilis also has such virulence factors, including emm homologs. When S. dysgalactiae ssp. equisimilis had species status, it was often referred to as “the human C.”
Subspecies dysgalactiae (Figure 5-3) infects the bovine mammary gland, originating in the mouth and vagina, and in skin lesions on the udder, producing sporadic cases of acute bovine mastitis; initiation of infection requires only a very low infectious dose, and it often occurs in synergy with Arcanobacterium pyogenes. These organisms also are a cause of arthritis, septicemia, and meningitis in goats and lambs. Human group G strains resembling S. dysgalactiae can also cause mastitis in cattle. Little is known about the virulence of S. dysgalactiae ssp. dysgalactiae, although most strains produce fibronectin-binding protein, streptokinase, and hyaluronidase. The role of M proteins in virulence of S. dysgalactiae ssp. equisimilis is not known, although at least four M types are produced.
Other strains in S. dysgalactiae ssp. dysgalactiae cause abortion, dermatitis, and septicemia in cattle and dogs, and abortion and a strangleslike syndrome in horses. It is perhaps most common in piglets; organisms carried by the sow infectby way of wounds, the umbilicus, or tonsil and produce suppurative arthritis.
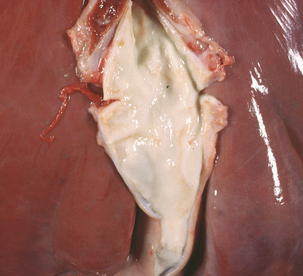
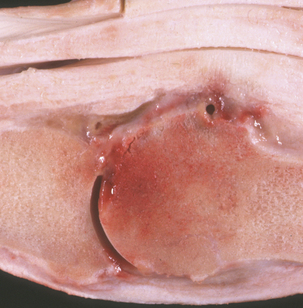
Streptococcus equi ssp. equi (group C) causes strangles, as well as genital and mastitic infections, in horses. It has also been associated with sup-purative omphalophlebitis in foals (Figure 5-4). Antigenic and genetic variations do not occur in S. equi ssp. equi, whereas the more cosmopolitan S. equi ssp. zooepidemicus is genetically heterogeneous. Survival of S. equi ssp. equi outside the host is limited (perhaps as much as 2 months under optimum conditions), so its distribution follows that of equids.
Partially immune animals (for example, those with residual maternal immunity or with a previous infection) may develop a catarrhal infection, with cough, fever, and mild nasal discharge. Lymph node abscessation is uncommon.
Strangles is a highly communicable infection of the upper respiratory tract and associated lymph nodes. The asymptomatic carrier state in horses is apparently quite rare, and the most important source of infection is a clinically affected horse shedding the organism in nasal discharges or from a draining abscess. Encapsulated abscesses, which later rupture to initiate shedding, may be a means of introducing the disease into a herd. The incubation period can be as short as 3 days or as long as 2 weeks. Streptococcus equi ssp. equi attaches to tonsillar crypt cells and adjacent lymphoid nodules. Within a few hours the organism translocates into the local lymphatics and cannot be detected on the mucosal surface. The influx of neutrophils is, in sum, inefficacious in phagocytosing and killing the organisms, so smears of lesion material reveal large numbers of streptococci in chains among degenerating neutrophils. Affected horses manifest fever, malaise, nasal discharge, cough, and swelling associated with the mandibular lymph node. Progressive lymph node abscessation causes pressure on the airway and respiratory difficulty, giving rise to the common name of the disease. Metastatic abscessation, with spread via circulation or the lymphatic route to lungs, abdomen, or brain, is not uncommon. The clinical course is usually short and uneventful, but myocarditis and purpura hemorrhagica can follow; immune complex formation can lead to acute leukocytoclastic vasculitis and glomerulonephritis.
Virulence factors of S. equi ssp. equi include a nonantigenic hyaluronic acid capsule, hyaluron-idase, SLO, streptokinase, IgG Fc-receptor proteins, peptidoglycan, and the antiphagocytic M protein. Isolate-to-isolate variation in virulence is related, for the most part, to the amount of antiphagocytic M protein produced. M proteins project as fibrils from the cell wall surface, and are composed of a coiled central region flanked by a short, coiled sequence at the N-terminus and a highly conserved region of hydrophobic and charged amino acids at the C-terminus. Its antiphagocytic activity lies in its ability to bind fibrinogen and complement control factor H, masking bacterial C3b-binding sites and inhibiting C3 and C5 convertases. Antibodies against N-terminal epitopes are opsonizing. Streptococcus equi ssp. equi M protein is uniform in size and antigenic properties, unlike those of S. pyogenes or S. equi ssp. zooepidemicus. In vitro passage results in diminished M protein production.
Isolates from cases of strangles produce mucoid colonies, indicative of encapsulation; virulence is reduced, but not abrogated, by loss of capsule. Peptidoglycan of S. equi ssp. equi activates complement by the alternate pathway, generating neutrophil chemotactic factors, and induces release of pyrogenic cytokines (such as interleukin [IL]-6). This accumulation of neutrophils at the site of infection and the febrile response contributes to the characteristic clinical signs of strangles.
Streptococcus equi ssp. equi also has Fc receptors on its surface, similar to protein A of Staphylococcus aureus, hypothetically allowing the organism to avoid opsonization by binding of the Fc portion of the antibody.
Streptokinase acts on the serine protease domain of equine plasminogen, forming plasmin, which in turn hydrolyses fibrin. Thus streptokinase may aid in dissemination of S. equi ssp. equi in tissue. SLO is produced, but its role in virulence of S. equi ssp. equi for horses has not been investigated.
Streptococcus equi ssp. zooepidemicus (group C) has a high degree of DNA sequence homology with S. equi ssp. equi. Streptococcus equi. ssp. zooepidemicus is a mucosal commensal, especially in the horse, but it is promiscuous in its selection of hosts, causing opportunistic respiratory, wound, and genital infections in most species. It is a frequent cause of mastitis in cattle and goats. Lambs infected with S. equi ssp. zooepidemicus can develop wound infections, pneumonia, and septicemia; puppies may be similarly affected. Chicken infections manifest as septicemia, and guinea pigs in rodent colonies develop epidemic lymphadenitis. Equine wound infections are quite common, as are joint ill, lymphatic abscesses approximating those caused by S. equi ssp. equi, and pneumonia in foals (Figure 5-5). Streptococcus equi ssp. zooepidemicus is the most common pathogen from the mare reproductive tract. Unlike S. equi ssp. equi, S. equi ssp. zooepidemicus is recovered from human infections, most of which follow consumption of contaminated dairy products.
< div class='tao-gold-member'>