Chapter 23 The Genera Mannheimia and Pasteurella
Mannheimia and Pasteurella are members of the class α-Proteobacteria, order Pasteurellales, family Pasteurellaceae, that contain small, facultatively anaerobic, nonmotile, gram-negative rods or coccobacilli that do not form endospores. Glucose is fermented without gas production, the oxidase reaction is normally positive, and nitrate is usually reduced to nitrite. These organisms are found as mucosal commensals of the oropharynx and gastrointestinal tract of healthy mammals, birds, and reptiles. They survive poorly outside the host.
THE GENUS MANNHEIMIA
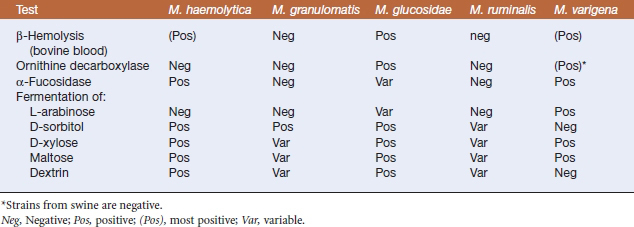
The new genus Mannheimia was established in 1999 to include trehalose-negative members of the Pasteurella haemolytica complex. All strains in the genus ferment mannitol, and failure to fermentD-mannose is a key by which Mannheimia spp. are differentiated from members of the genus Pasteurella. The five current species in the genus Mannheimia are Mannheimia haemolytica, Mannheimia granulomatis, Mannheimia glucosida, Mannheimia ruminalis, and Mannheimia varigena (Table 23-1).
TABLE 23-1 Hosts and Significance of Mannheimia Species
| Species | Host(s) | Significance |
|---|---|---|
| M. haemolytica | Cattle | Pneumonia |
| Sheep | Pneumonia, septicemia, mastitis | |
| M. granulomatis | Cattle | Panniculitis (lechiguana) |
| Deer and hares | Bronchopneumonia, conjunctivitis | |
| M. glucosida | Sheep | Normal respiratory flora |
| M. ruminalis | Cattle, sheep | Normal ruminal flora |
| M. varigena | Pigs | Septicemia, enteritis, pneumonia |
| Cattle | Septicemia, pneumonia, mastitis |
DISEASE AND EPIDEMIOLOGY
Mannheimia haemolytica is one of the most important pathogens of domestic cattle. It is the primary bacterial agent responsible for bovine pneumonic pasteurellosis, also known as “shipping fever,” because of its frequent occurrence in transported animals. It is a major cause of morbidity and mortality, accounting for approximately 30% of the total cattle deaths globally. A recent study estimated the annual economic loss to the U.S. beef cattle industry at $640 million.
PATHOGENESIS
The primary virulence factors are leukotoxin and lipopolysaccharide (LPS). Leukotoxin, a pore-forming cytotoxin of the RTX family, induces lysis of ruminant leukocytes and platelets, but not those from other species. It impairspulmonary macrophage function and bacterial clearance and damages lung parenchyma through the release of proteolytic enzymes from lysed leukocytes. Decreased antigen-presenting capacity of macrophages aids in lung colonization, directly impairing induced pulmonary defenses.
Other virulence determinants of M. haemolytica include capsular polysaccharide, iron-regulated proteins, enzymes, and fimbriae (Table 23-2). Antibodies against a carbohydrate surface component of M. haemolytica may also protect against M. haemolytica infection, suggesting a role in virulence for this yet-to-be characterized factor.
TABLE 23-2 Virulence Factors of Mannheimia haemolytica
| Virulence Factor | Activity |
|---|---|
| Leukotoxin | Pore-forming cytolysin, lethal for leukocytes and platelets; impairment of pulmonary macrophage function |
| Lipopolysaccharide | Stimulates production of proinflammatory cytokines, lipid mediators, procoagulant substances, oxygen radicals, proteases that damage lung parenchyma |
| Capsular polysaccharide | Adherence to lower respiratory mucosa, inhibits phagocytosis, mediates resistance to complement-mediated lysis, neutrophil chemoattractant |
| Fimbriae | Adherence |
| Siderophore | Iron acquisition |
| O-sialoglycoprotein endopeptidase | Cleaves glycoproteins, inactivates macrophages and other leukocytes |
| Neuraminidase | Reduces viscosity of respiratory mucus, impairs mucociliary blanket, allows bacterial penetration to respiratory epithelium |
DIAGNOSIS
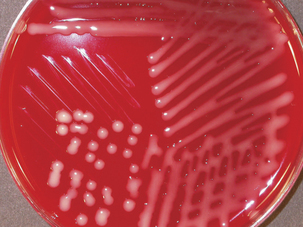
Diagnosis is based on bacterial isolation from clinical specimens. Mannheimia spp. grow readily on blood agar or glucose agar plates supplemented with serum. Colonial morphology is similar forall species; colonies are smooth, grayish, variablyβ-hemolytic, and 1 to 2 mm in diameter after24 hours’ incubation (Figure 23-1). Species are differentiated on the basis of phenotype (Table 23-3).
PREVENTION AND CONTROL
A commercial vaccine containing leukotoxoid and surface antigens protects against pneumonic pasteurellosis. Commercial bacterins are, in general, inefficacious and may actually exacerbate disease. Vaccination with bacterins induces formationof opsonizing antibodies that facilitate phagocytosis, leading to increased phagocytic cell lysis andsubsequent severe pulmonary inflammation. Antiinflammatory agents may be useful, and anti-microbials (macrolides, penicillins, cephalosporins, or florfenicol) are usually administered to prevent further bacterial multiplication and secondary infection. Plasmid-mediated resistance in some strains may be a significant problem.