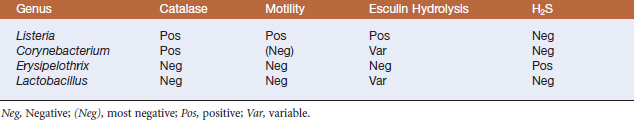
The natural habitat of members of the genus Listeria is probably decomposing plant matter, where they live as saprophytes. However, they are isolated from many environmental sources, and ruminants probably maintain environmental populations by continuous fecal-oral enrichment.
Until the mid-1980s, L. monocytogenes was, to human medical microbiologists, nothing more than a part of the arcane province of veterinary microbiologists and a model organism for study of the cell-mediated immune response. However, in 1985, listeriae in general moved to national prominence because of their role in extensive outbreaks of food-borne disease in humans. Listeria monocytogenes remains one of the most important causes of human food-borne illness; overall, it is the fifth most common cause of bacterial meningitis in the United States, but is second most important in neonates and in individuals older than age 60.
In the veterinary arena, L. monocytogenes is still a common pathogen; listeriosis has been described in more than 40 species of animals, but it is particularly in domestic ruminants. It causes central nervous system (CNS) infections in cattle, sheep, goats, horses, fowl, and dogs, as well as abortions and mastitis in cattle, sheep, and goats. Systemic listeriosis occurs in laboratory rodents, poultry, swine, cattle, and sheep. It is not uncommon asa cause of abortions and septicemia in horses.
Our understanding of the pathophysiology of listeriosis is mainly a synthesis of epidemiologic, clinical, and pathologic findings in natural cases and study of experimental infections in laboratory rodents; statements about events occurring in humans and domestic animals before association of L. monocytogenes with epithelial cells are mainly based on interpolation and extrapolation.
Disease in monogastrics is often mild or even asymptomatic. It commonly manifests as febrile gastroenteritis, a mild, influenza-like disease. More serious effects of infection occur in the form of invasive disease, especially in patients who are immunocompromised (in particular, those suffering from acquired immunodeficiency syndrome [AIDS], diabetes, or alcoholism). Renal transplantation and lymphoreticular malignancies are also associated with increased risk of infection. Such individuals develop meningoencephalitis, often accompanied by an ultimately fatal bacteremia.
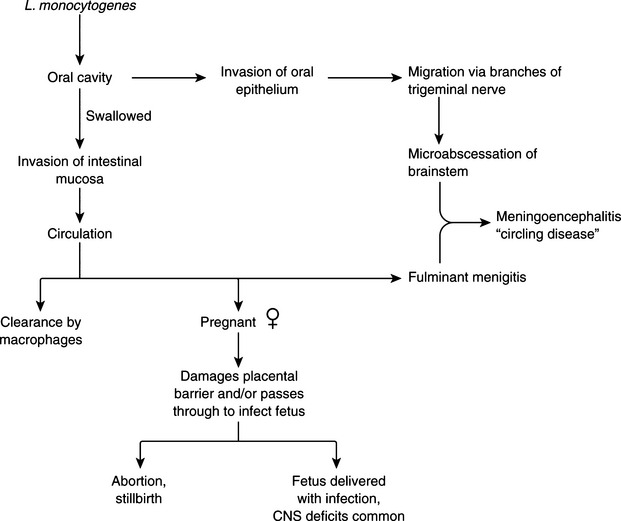
Listeria monocytogenes–associated puerperal sepsis in humans is a flulike illness that occurs during pregnancy. Many cases in the 1985 food-borne disease outbreak in the southwestern and western United States were in pregnant women, most with disastrous consequences for the fetus. The organism is usually ingested and after crossing the intestinal mucosal barrier, causes bacteremia. It crosses the placenta (Figure 10-1), causing in utero fetal infection, resulting in stillbirths, preterm labor, and the birth of infants with systemic infection. Neonatal meningeal sepsis caused by L. monocytogenes occurs in two forms. Early onset disease is predominantly septicemic, with affected infants having low birthweight. These cases are often associated with obstetric complications and colonization of the maternal genital tract by L. monocytogenes. Late-onset disease manifests mainly as meningitis and occurs inchildren of normal birthweight, usually without obstetric complications or genital tract colonization. Outside the neonatal period, the incidence of neural listeriosis increases with advancing age.
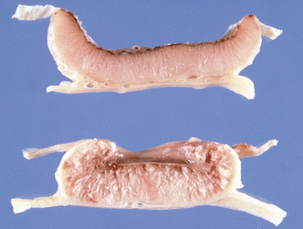
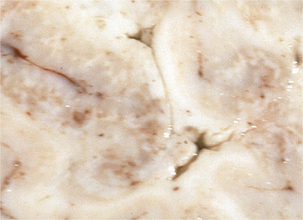
Disease in domestic ruminants mainly takes the form of meningoencephalitis, called circling disease in its most common form (Figure 10-2). The organism is found commonly in poor-quality silage (pH ≥5). Affected animals circle, in one direction only, and display unilateral facial paralysis, difficulty in swallowing, fever, blindness, and head pressing; paralysis and death follow in 2 to 3 days. If treated, less than 40% of animals will recover, most with permanent brain damage (Figure 10-3).
In pregnant animals, L. monocytogenes may localize in the placentomes and cross over to amniotic fluid. It multiplies there and is ingested by the fetus, eventually causing fetal death and abortion. Listerial abortion usually occurs in late gestation.
Although ingestion seems to be the most common route of infection, L. monocytogenes can also enter via the nasal mucosa and conjunctivae. It may find direct access to the nervous system by way of the dental plates of the trigeminal ganglia.
In milking cows, the mammary gland can become involved, resulting in subclinical mastitis and contamination of milk. The organism may then be spread to humans in unpasteurized dairy products; L. monocytogenes may also survive low-temperature pasteurization from its place of residence inside macrophages, and its lengthy survival in nature makes postpasteurization contamination a continual threat. The ability of L. monocytogenes to grow at 4° C is useful in diagnosis (see p. 92), but is an additional food-borne disease risk, in that the organism can multiply during production, storage, shipping, and marketing of dairy products.
Comparison of endemic and epidemic rates for human listeriosis suggests that in the gastroenteric form of infection, L. monocytogenes has lower virulence than other food-borne pathogens. This is consistent with the relatively high oral LD50 rates in mice and the large numbers often detected in outbreak-associated foods. However, the lengthy incubation period preceding invasive disease (suggesting amplification of a small inoculum), as well as the expectation of strain-to-strain variability in virulence and differences in host susceptibility, suggest that the infectious dose need not always be large.
Listeriosis can be usefully modeled in rodents and in cell cultures. As noted, murine listeriosis has been an important model of the cell-mediated immune response to bacteria; discovery of the activated macrophage response, as well as natural killer cells and cytotoxic T-cell activity against intracellular organisms came from the study of L. monocytogenes infection in mice. The mouse model has also been used extensively in the study of pathogenesis. Infection by oral or intraperitoneal routes, in pregnant or nonpregnant conventional or gnotobiotic mice, has provided an exceptional platform for investigation of invasion, intracellular survival, and general pathogenesisof listeriosis. Listeriae invade intestinal and macrophage cell lines and produce plaques in fibroblast monolayers; much of our knowledge of cell invasion, intracellular survival, and cell-to-cell spread comes from studies in cultured cells.
As noted, exposure to listeriae is primarily by ingestion. In its gastroenteric form, invasion beyond the mucosa is limited, but when L. monocytogenes crosses this barrier, the liver is probably the first target organ. Multiplication there is eventually controlled by a cell-mediated immune response, which is probably maintained in most individuals by continual exposure to listerialantigens. Low-level bacteremia may result from uncontrolled multiplication in the liver of debilitated and immunocompromised patients, allowing invasion of brain and gravid uterus.
Virulence of L. monocytogenes is mediated by many factors. Flagella-mediated motility is unlikely to be important virulence factor, although actin-based motility is very important (see later). Little is known about adherence of the organism to target cells, although bacterial α-D-galactose residues may bind to receptors on intestinal cells.
Listeria monocytogenes and Listeria ivanovii arefacultative intracellular parasites, surviving in macrophages and invading nonprofessional phagocytes, including epithelial cells, hepatocytes, and endothelial cells. M cells may be a target in vivo, although the organism may enter intestinal crypt cells, the only undifferentiated mucosal cells. Cellular uptake of listeriae is by induced phagocytosis, mediated by listerial membrane proteins called internalins. Specific mutation in the structural gene, inlA, eliminates invasion, and this deficit can be complemented by supplying inlA in trans. InlA has sequence similarity with the M protein of Streptococcus pyogenes, although functionally it stimulates, rather than inhibits phagocytosis, and may interact in similar ways with host proteins that have shared domains but dissimilar functions. Cell-to-cell spread, in cell monolayers or in the endothelium in vivo, apparently does not involve internalins.
Pathogenic listeriae have an intracellular life cycle involving escape from the phagosome, intracytosolic multiplication, actin-based motility, and lateral spread to adjacent cells. After entering epithelial cells, L. monocytogenes escapes the phagosome and multiplies in the cytoplasm. Exocytosis from the epithelial cell is followed by phagocytosis, by macrophages and neutrophils; multiplication in these cells is eventually lethal, and is followed by secondary phagocytosis and, in some proportion of infections, systemic spread. Beyond the epithelium, relatively little is known about pathogenesis of listeriosis.
The major virulence factor mediating intracellular survival is a cholesterol-binding cytolysin called listeriolysin (LLO). It shares 40% to 50% amino acid sequence similarity to other members of a family, sometimes referred to as thiol-activated toxins, and including streptolysin O, pneumolysin, perfringolysin, alveolysin, pyolysin, and others. Common characteristics of this group are binding to cholesterol in cell membranes and oligomer-ization and insertion to form a transmembrane pore. An undecapeptide near the C-terminus is involved in these activities, as well as reversible inactivation by oxygen in most members of the group.
LLO is produced in varying quantities by all virulent strains of L. monocytogenes. Its importance in the process of invasion and spread of the organism is in its facilitation of escape from the phagocytic vesicle. The LD50 of LLO mutants is5 logs higher than that of the wildtype, and the mutants do not survive in macrophages. Bacillus subtilis (which, in the wildtype, does not survive intracellularly) engineered to express LLO escapes the phagocytic vesicle and enters the cytoplasm of macrophages.
Regulation of production of LLO production and activity are important in that L. monocytogenes prefers to maintain intracellular residence; producing LLO at the same rate and with the same activity after phagosomal escape would be roughly the equivalent of an arsonist taking his work home. In fact, the rate of synthesis and secretion of LLO by extracellular bacteria (which includes the interior of the phagosome) is approximately 50 times higher than cytosolic bacteria, which secrete approximately 1 molecule per bacterium per minute. LLO is a major protective antigen, giving rise to major histocompatibility complex (MHC) class I–restricted cytotoxic T-lymphocytes (CTLs) in mice; it is immunodominant because it is rapidly degraded and efficiently processed into an MHC class I–associated epitope.
Listerial phospholipases also participate inthe interaction of L. monocytogenes with cells. A phosphatidylinositol-specific (called PI-PLC, and encoded encoded by plcA) and a so-called broad-activity phospholipase C (PC-PLC, encoded by plcB) can hydrolyze most phospholipids in host cells. PI-PLC mutants are less efficient than wildtype organisms in phagosomal escape, which may account for the limited replication of plcA mutants in mouse peritoneal macrophages. PC-PLCis apparently involved in cell-to-cell spread of L. monocytogenes, enabling the organism to escape from the double-membraned vesicle in which it is enclosed after moving from one cell to another. Thus the functions of the two phospholipase Cs overlap during intracellular infection.
Many virulence genes of L. monocytogenes are regulated by a protein, PrfA, that may bea temperature sensor; genes controlled by prfA are expressed to a greater extent at 37° C than at lower temperatures, suggesting upregulation when the organism comes into contact with a mammalian host.
Bacteria, freed of the phagosome and living in the cytosol, polymerize actin, forming a tail and facilitating motility. A hollow mesh, formed on the bacterial surface, is left behind as the organism moves forward; actin is depolymerized and thus turned over. With this propulsion system, L. monocytogenes can move through the cytoplasm at the blistering pace of 1.5 μm/second. Actin polymerization is mediated by a bacterial protein, ActA, that localizes to the end of the organism on which the tail forms.
Bacteria encountering a plasma membrane continue to move forward, producing protrusions that extend into the adjacent cell. This eventually progresses to release of the organism in a double-membraned vesicle (one for each cell membrane); escape from this vesicle involves the action ofPC-PLC, as mentioned. This lateral spread of the organism is seen in the plaques produced in monolayers of cultured cells. In vivo it probably serves to increase the population of listeriae, which escape to the gut lumen, returning toa saprophytic lifestyle, or are exocytosed on the basolateral surface of the epithelium, to participate in possible systemic disease.
Listeria ivanovii behaves in much the same manner as L. monocytogenes, except that it lacks the extraordinary degree of promiscuity in host selection; it is exclusively (or nearly so) a pathogen of ruminants. Human cases are rare. Other species, such as Listeria seeligeri, Listeria grayi, Listeria innocua, and Listeria welshimeri, may occasionally be isolated from clinical specimens (including at least one case of human listeriosis due to L. seeligeri), but they are considered to be soil contaminants.