Chapter 40 The Family Anaplasmataceae
The family Anaplasmataceae has beenreorganized based on 16S rRNA gene sequences to include all species ofα-Proteobacteria in the genera Neorickettsia, Anaplasma, Ehrlichia, and Wolbachia; Aegyptianella has been retained as a genus incertae sedis (Table 40-1). Wolbachia spp. are associated exclusively with invertebrates, and the genera Eperythrozoon and Haemobartonella have been transferred to the order Mycoplasmatales. The obligately intracellular Anaplasmataceae replicate within cytoplasmic vacuoles of host cells, such as erythrocytes, reticuloendothelial cells, bone marrow–derived phagocytic cells, endothelial cells, and reproductive tissues of insects, arthropods, or helminths. Most members have a trematode, tick, or other invertebrate vector host.
THE GENUS NEORICKETTSIA
Neorickettsia helminthoeca
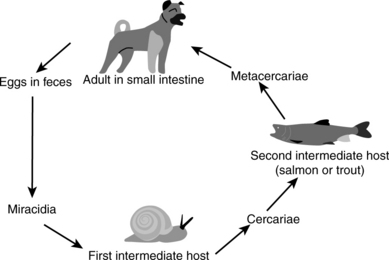
Salmon poisoning disease was first recognized in the early nineteenth century by settlers in the Pacific Northwest when their dogs fell ill after consuming raw salmon. The causative agent, N. helminthoeca, was characterized in 1953, but could not be placed in a previously described genus based on the disease and morphologic characteristics. It is unique, in that it is the only obligately helminth-borne pathogenic bacterium. Canine mononuclear cells are infected after dogs ingest salmonid fish encysted with a fluke, Nanophyetus salmincola (Figure 40-1), infected with the organism. The disease is indigenous to river areas of the U.S. Pacific Northwest coast, from northern California to southwestern Washington State. Occasional cases associated with fish migration have been reported in British Columbia.
Salmon poisoning (Figure 40-2) generally affects only members of the family Canidae, but death of captive polar bears has been reported in association with salmon poisoning disease. After ingestion of infested fish, the trematodes attach to and penetrate deeply into the mucosa, particularly in the duodenum but also throughout the small and large intestines. The precise mechanism of infection remains to be elucidated, but superficial enteritis develops rapidly, and progresses to hemorrhagic enteritis. Bacteria spread by the hematogenous route to lymph nodes, spleen, liver, lungs, brain, and thymus. Acute disease is characterized by fever, depression, dehydration, anorexia, diarrhea, and lymphadenopathy. The case fatality rate in untreated dogs is 50% to 90% and the exact cause of death remains unknown. Recovered animals are immune to reinfection.
Antemortem diagnosis is based on detection of fluke eggs in feces, presence of compatible clinical signs, history of travel to the Pacific Northwest, demonstration of rickettsiae in lymph node aspirates, and serology. Trematode eggs are operculated and are passed in the feces 5 to 8 days after fish ingestion. They can be detected by direct smears or sugar flotation techniques. Macchiavello or Giemsa stains of lymph node aspirates reveal intracytoplasmic rickettsial bodies, but organisms are not detected by microscopic examination of circulating lymphocytes. Affected animals typically seroconvert 13 to 15 days postinfection, so several veterinary diagnostic laboratories offer serologic diagnosis by way of indirect fluorescent antibody or complement fixation tests.
Neorickettsia (Ehrlichia) risticii
Neorickettsia risticii appears to be maintained in nature in a complex aquatic ecosystem. The infection cycle apparently involves an intermediate snail reservoir and a trematode cercarial vector. In the laboratory, researchers have infected mice and horses by intraperitoneal subcutaneous inoculation, respectively, with trematodes from snails. DNA of N. risticii has been found in virgulatecercariae from the freshwater snails Juga yrekaensis in northern California and Elimia livescens in Ohio. Such DNA has also been detected in metacercariae from aquatic insects, such as caddisflies, stoneflies, damselflies, mayflies, and dragonflies. Oral transmission of PHF has been demonstrated in horses fed caddis flies, and it is natural to speculate that transmission to horses involves accidental ingestion of insects harboring infected metacercariae. Potential helminth vectors include Lecithodendrium and Acanthatrium spp. These N. risticii–infected helminths have been found in the intestinal tracts of bats and birds, but no definitive reservoir host has been identified. Besides horses, other susceptible mam-mals include cattle, mice, dogs, and cats. In endemic locations, antibody titers to N. risticii have been found in goats, pigs, cats, dogs, and coyotes.
THE GENUS ANAPLASMA
Anaplasma marginale
The severity of the disease is related directly to the proportion of the erythrocyte mass destroyed. Hemoglobinuria does not occur in anaplasmosis because the destruction of erythrocytes occurs intracellularly rather than intravascularly. Serum factors sensitize erythrocytes to phagocytosis by the monocyte-macrophage system and these opsonins increase in the circulation before the hemolytic crisis. Fetal hypoxia is responsible for abortion.
A presumptive diagnosis of anaplasmosis is based on clinical signs and hematologic findings in animals in an endemic area. Serologic testing, direct examination of blood smears, and molecular methods are also useful. Rapid card agglutination, complement fixation, and enzyme immunoassays are available for serologic diagnosis. Phenotypic criteria for identification of ruminant erythrocytic Anaplasma spp. (A. centrale, A. marginale, and A. ovis) have for many years relied on subjective methods, such as the location of inclusion bodies in host red blood cells (Figure 40-3) and host pathogenicity (cattle vs. sheep). Recentstudies have confirmed the suitability of 16S rDNA sequence analysis to define the species from blood samples.
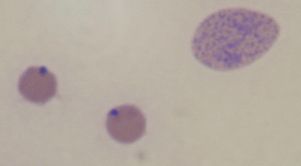
FIGURE 40-3 Blood smear from cow with anaplasmosis, showing two typically parasitized erythrocytes and an immature form.
(Courtesy Raymond E. Reed.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree