Chapter 19 THE EARLY PREGNANCY
INTRA-OVIDUCTAL EMBRYO DEVELOPMENT
At ovulation, the oocyte is released into the relatively small oviductal fimbria and descends to the ampullary region where, if capacitated sperm are present, fertilization will take place.1,2 Once fertilized, the newly formed “zygote” embarks on a series of regular cell divisions, such that it reaches the two-cell stage within 24 hours, four to six cells within 48 hours, and by 72 hours contains eight to ten cells.3 Between days 4 and 5, a “morula” is formed, and the as yet undifferentiated cells “compact” into a homogenous ball until late on day 5 or early on day 6 when the embryo starts to develop into a “blastocyst” (i.e., to form a central cavity while the constituent cells differentiate visibly into either inner cell mass [ICM] or trophectoderm). At around the same time, day 6–7 after ovulation, the embryo finally enters the uterus,4,5 generally at the late morula or early blastocyst stage of development,6 containing around 600 cells7 and still surrounded by its original protective coat, the zona pellucida.4,6 As a result of the recent increase in commercial embryo transfer (ET), it has become apparent that embryonic development up to the blastocyst/uterine entry stage can be retarded by as much as 1–2 days by factors such as advancing maternal age, use of frozen-thawed semen, and insemination early in the breeding season,8 or by in vitro embryo production9,10; this needs to be taken into account when attempting to recover embryos for immediate transfer or cryopreservation, and when selecting a suitably synchronized recipient for ET.
Selective Oviductal Transport
In the horse, only developing embryos are transported into the uterus; unfertilized oocytes (UFOs) instead lodge in the ampulla of the oviduct where they slowly degenerate.11,12 Although UFOs will occasionally enter the uterus along with an embryo from the same or a subsequent cycle,13 independent transport of a UFO to the uterus is very uncommon. For this reason, if only a UFO is identified during an embryo flush, it is advisable to recheck the lavage equipment and/or repeat the flush in the expectation that the oocyte accompanied an embryo into the uterus.8
In an elegant series of experiments by Weber et al.,14–17 the oviduct’s ability to differentiate between fertilized embryos and UFOs was shown to be based not on any subtle fertilization-induced difference in shape or zona pellucida composition, but rather on embryonic secretion of prostaglandin E2 (PGE2). In short, Weber et al.14,15 demonstrated that equine embryos not only secrete PGE2 from around day 4–5 after ovulation, but that continuous local (intra-oviductal) administration of PGE2 is sufficient to induce premature uterine entry of embryos.15 Weber et al.17 summarized their findings by proposing that a developing embryo lodges at the ampullary-isthmic junction as a result of tonic contraction of the isthmic circular smooth muscle until PGE2 secretion by the compact morula is sufficient to relax the isthmic “sphincter” and allow the embryo to pass rapidly into the uterus. Robinson et al.18 made use of these discoveries to develop a technique for hastening oviductal passage so that embryos could be collected reliably by uterine lavage at a stage of development when they were, for example, still suitable for cryopreservation; laparoscopic application of PGE2 gel to the oviduct on day 4 after ovulation allowed embryos to be recovered from the uterine lumen 24 hours later. This technique has not, however, been adopted in clinical practice, largely because laparoscopic administration requires the mare to be starved, requires her flank to be surgically prepared, and involves entry into the abdominal cavity; all of these reduce its appeal to the owners of valuable mares.19
Early Pregnancy Factor
Although selective oviductal transport is an important example of early embryo-maternal interaction, it does not result in any immediate, measurable changes in maternal physiology that could serve as the basis of an early pregnancy test. On the other hand, as in other species, there have been reports of an “early pregnancy factor” (EPF) that can be detected in the serum of pregnant mares from as early as day 2 after ovulation using the Rosette Inhibition Test (RIT).20 Although the precise identity and function(s) of the equine EPF are not known,21 it is clear that a reliable and practical EPF assay would improve our ability to examine the scale and timing of embryonic death, investigate unexplained infertility, and improve the efficiency of ET programs in particular, and breeding programs in general. Whereas the RIT is too laborious and expensive to be of use in practice, an “early conception factor” test that aims to detect the EPF protein in blood serum has been marketed for mares inseminated 3–30 days previously (Horse ECF cassette test, Concepto Diagnostics, Knoxville, TN). Unfortunately, early field trials indicated a high incidence of false positives in known non-pregnant mares, and a general low ability to discriminate between pregnancy and non-pregnancy.22 for these reasons, the ECF test is not currently recommended for determining pregnancy status in mares.
Embryonic Death During the Oviductal Period
Early pregnancy loss is a common and economically important problem in brood mares. However, pregnancy loss during the oviductal period appears to be primarily a cause of reduced breeding success in aged mares, and much less common in young, fertile mares.23 Indeed, while fertilization rates in experimental settings are similarly high in both aged and young mares (approaching 90%23), embryo recovery rates at days 4 and 7 after ovulation are significantly lower in older mares,23–25 suggesting significant early losses in the latter group. Furthermore, the embryos recovered from older mares are significantly less likely to result in an ongoing pregnancy after transfer to a fertile recipient. There are, of course, two major possible contributors to embryonic loss during the oviductal period, namely an inadequate oviductal environment, or intrinsic abnormalities of the embryo arising from a defective oocyte or a defective sperm. Since equine embryos spend an unusually long part of their early development within the oviduct, it is probable that an inadequate oviductal microenvironment— for example, due to salpingitis (which is more common in aged mares26) or impaired oviduct secretory activity—would adversely affect embryonic survival. On the other hand, both oviductal pathology and insufficiency are difficult to diagnose or treat and, furthermore, oocyte-transfer (OT) experiments suggest that embryonic abnormality arising from age-related poor oocyte quality is by far the most significant contributor to intra-oviductal embryonic death.27 The significance of embryonic abnormalities to EPL will be discussed further in the section relating to EPL during the early intra-uterine period.
EARLY INTRAUTERINE DEVELOPMENT: THE PRE-FIXATION PERIOD
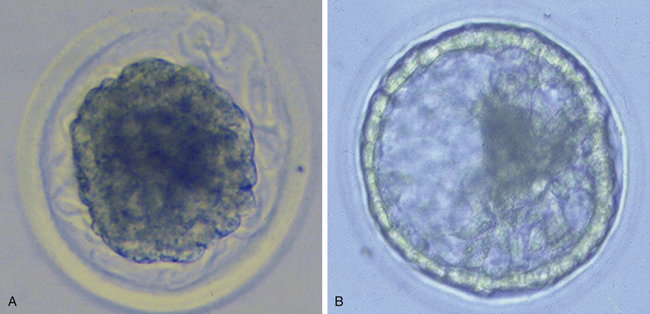
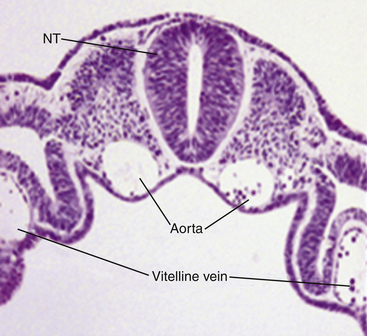
Very soon after its arrival in the uterus, the equine early blastocyst begins to form an acellular glycoprotein “tertiary embryo coat,” the so-called “blastocyst capsule.”28 The capsule first becomes visible as patches of iridescent material between the trophectoderm and the zona pellucida of the late morula/early blastocyst; these patches soon coalesce to form a confluent layer by about day 7 after ovulation, when blastocyst formation is complete (Fig. 19-1).29 The blastocyst then embarks on a period of rapid expansion during which it sheds its zona pellucida to emerge fully enveloped by a complete capsule; at this stage (i.e., day 7 after ovulation) the embryo has a diameter of approximately 250 μm6 and contains a few thousand cells.7,9 During the following week, the conceptus remains spherical and continues to expand rapidly, reaching 3–5 mm in diameter (and in excess of 45,000 cells) on day 10 after ovulation and 15–20 mm by day 14. Expansion is predominantly a result of yolk sac fluid accumulation6 and is accompanied by a corresponding increase in the size and dry weight of the capsule, which remains as a protective envelope around the conceptus.6,30 During the same period, the blastocyst cavity becomes lined with primitive endoderm to form a true “yolk sac” and the small ICM expands and develops to form the bi-layered embryonic disc (trophectoderm and yolk-sac endoderm). Between days 10 and 12, the embryonic disc becomes macroscopically visible and at around day 12 the primitive streak, the precursor of the embryo proper, becomes microscopically visible (Fig. 19-2); subsequently, cells migrate through the primitive streak and differentiate to create mesoderm in a process known as gastrulation, which heralds the formation of a recognizable multi-cellular embryo.31,32
One of the defining characteristics of pre-fixation horse conceptus development is the presence of the blastocyst capsule. Although the exact functions of the capsule are not known, its tough, elastic nature almost certainly allows it to provide mechanical protection (Fig. 19-3) to the delicate conceptus during the day 10–16 period when it is propelled around the uterine lumen by myometrial contractions.33 The proposed anti-adhesive properties of the capsular glycoproteins presumably further facilitate conceptus migration.30 Although not proven, the capsule has also been proposed to provide protection against microorganisms and the maternal immune system,34 and to act as an interface for storing or transferring molecules involved in conceptus-maternal communication (e.g., insulin-like growth factor binding protein 3 [IGF-BP3]35) or transfer of nutrients (e.g., uterocalin/P19).36,37
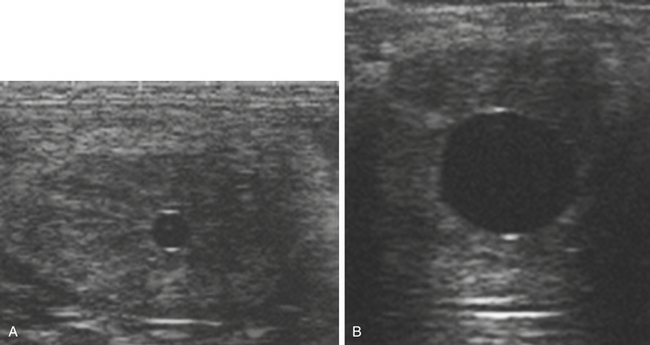
The blastocyst capsule also appears to be instrumental in maintaining the characteristic spherical form of the early equine conceptus,38 a feature that differs markedly to the rapid elongation seen in the other large domestic species at an equivalent stage of development (pigs and ruminants39). As discussed later, the maintenance of the spherical shape, together with an unusually prolonged period of intrauterine mobility, are linked to the manner in which the equine conceptus achieves “maternal recognition of pregnancy.” In addition, expansion of the conceptus as a sphere confers the considerable practical advantage of enabling ultrasonographic detection from as early as day 9–10 after ovulation.40 In practice, because detection of a 2-3–mm vesicle on day 10 after ovulation has a sensitivity of no more than 70%, routine scanning for pregnancy is generally delayed until at least day 12 after ovulation and is most commonly performed on days 14–16 when the conceptus is visible as an approximately 2-cm fluid-filled vesicle with characteristic hyper-echogenic artefacts known as “specular echoes” at the dorsal and ventral poles (Fig. 19-4).41,42 Indeed, day 16 after ovulation is late enough to definitively diagnose non-pregnancy and identify most asynchronous twins (e.g., even if a second ovulation took place 3–4 days after the first), while retaining plenty of time to plan mating at the subsequent spontaneous estrus, should that be necessary. Prior to the widespread use of ultrasonography, early pregnancy diagnosis was generally performed by strategic teasing or transrectal manual palpation of uterine and cervical tone, where the latter typically increases beyond those of normal diestrus from around day 16 after ovulation.43 And although manual pregnancy diagnosis alone is insufficient to either detect multiple pregnancy or EPL or to reliably differentiate between early pregnancy and prolonged diestrus, “palpation” is still an important part of determining whether a pregnancy is progressing normally.
Conceptus Migration
In the period following its arrival in the uterus, the equine conceptus does not form a stable attachment to the endometrium but instead remains mobile and migrates continuously throughout the uterine body and horns,40,44 propelled by myometrial contractions induced by prostaglandins produced either directly or indirectly (by stimulating local production in the endometrium) by the conceptus.33 The mobile phase eventually comes to an end when the combination of increasing conceptus size and the pregnancy-related increase in uterine tone cause the conceptus to lodge at the base of one of the uterine horns on day 16 or 17 after ovulation.45 Changes in capsule composition also appear to play a role in fixation, since fixation is accompanied by structural changes in the capsule (loss of sialic acid residues) consistent with a loss of anti-adhesive properties.46 The physiological function of conceptus migration in the mare appears to be two-fold: (1) to allow the conceptus to interact with, and send biochemical signals to, sufficient endometrial area to prevent luteolysis (i.e., to effect maternal recognition of pregnancy), and (2) to improve the ability of the growing conceptus to harvest nutrients from the uterine milk or “histotrophe,” in the period before the formation of a stable placental attachment (Fig. 19-5).38,47
Maternal Recognition of Pregnancy
Short48 coined the term maternal recognition of pregnancy (MRP) to describe the process by which a female mammal determines that a conceptus is present in her uterus and makes the appropriate physiological changes to ensure that an adequate environment is established for the growth and development of that conceptus. Since the primary determinant of an “adequate environment” is the continued supply of progesterone, MRP is now more commonly taken to refer specifically to the conceptus-initiated events that result in the prolonged lifespan and secretory activity of the primary corpus luteum (CL). In the horse, MRP appears to depend on an absolute suppression of the endometrium’s ability to release the luteolytic hormone prostaglandin F2α (PGF2α) in response to oxytocin49,50 secreted by either the hypothalamus-anterior pituitary unit51 or the endometrium itself.52–54 Since the oxytocin responsiveness that underpins cyclical luteolysis develops at around day 10 after ovulation,49,55 it is assumed that conceptus signaling to achieve MRP also begins at around this time. At the other extreme, pregnancy removal experiments56 suggest that signaling to complete MRP is not complete until at least day 14 after ovulation, and it is generally thought that MRP consists of a complex continuum of conceptus-maternal signaling events that together ensure prolonged CL survival.57
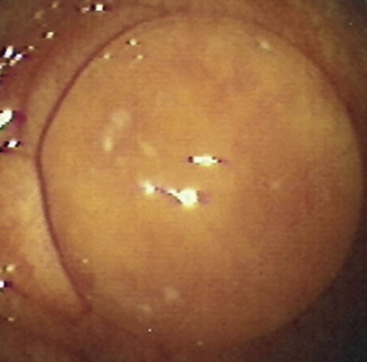
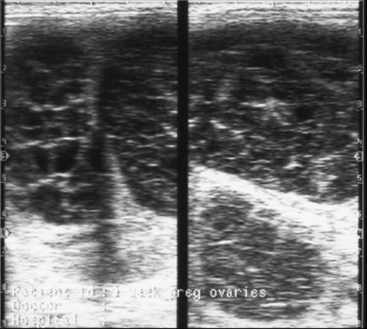
MRP in the mare is known to depend critically on conceptus migration, where the latter ensures that the anti-luteolytic signal is distributed (repeatedly) to all parts of the uterus, thereby allowing it to suppress PGF2α release from sufficient endometrium to avert luteolysis. Indeed, when McDowell et al.58 surgically restricted horse conceptuses to a single uterine horn, PGF2α release was not inhibited sufficiently to block luteolysis and the pregnancies failed. In practice, it is thought that large single intraluminal uterine cysts or local accumulations of cysts may present sufficient impediment to conceptus migration to endanger MRP, particularly if they are located at the base of a uterine horn; this is one of the reasons for recommending the removal of large intraluminal endometrial cysts when they are detected during a pre-breeding examination (Fig. 19-6).59
Given its critical importance in pregnancy maintenance and potential use for diagnosing pregnancy and detecting incipient pregnancy loss, it is surprising that the equine conceptus MRP signal has still not been identified. Molecules proven to function as MRP signals in the other large domestic species, such as interferon-τ in the ruminants and estrogens in the pig (for review, see Spencer et al.60), are either not secreted by equine conceptuses (interferon-τ: 57) or are secreted but do not reliably extend CL lifespan (estrogens33). Studies to investigate the size of the conceptus product that inhibits PGF2α secretion by equine endometrium incubated in vitro concluded that the equine MRP signal must have a molecular weight of 1-6 Kda61 or 3-10 Kda62; however, further characterization has proven elusive. On the other hand, MRP stage equine conceptuses have been shown to secrete a variety of hormones, including PGE2 and PGF2α,63 IGF-1,64 and estrogens65,66 that, although they may not be directly involved in inhibiting endometrial PGF2α secretion, almost certainly play significant roles in processes essential to pregnancy maintenance, such as conceptus migration,33 increased uterine vascularity,67 and qualitative and quantitative alterations in the composition of the uterine secretions that make up histotrophe.68
Early Management of Twin Pregnancy in the Mare
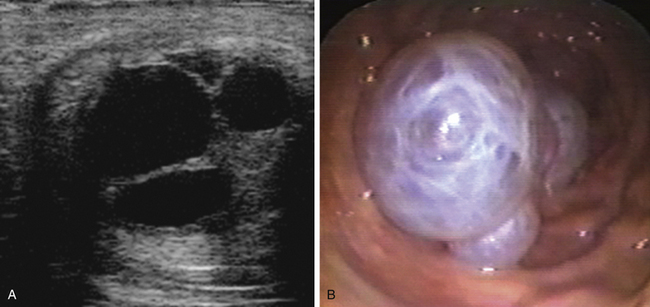
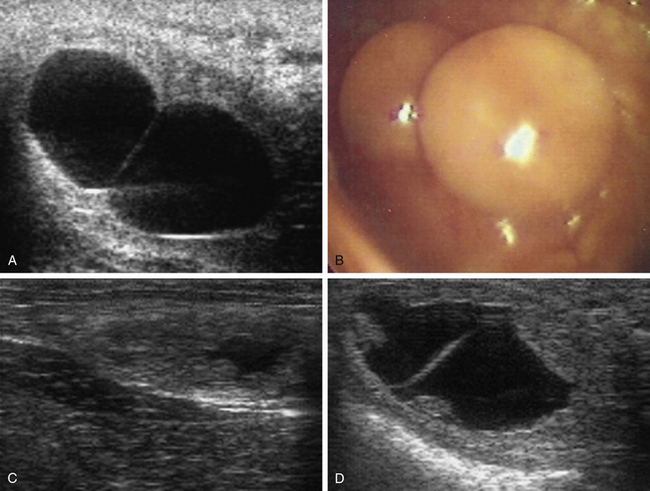
The conceptus mobile phase has proven to be of particular clinical use with respect to the management of twin or multiple pregnancy. Before the introduction of ultrasonography for the early detection of pregnancy in the 1980s,69,70 twin pregnancy was one of the most common causes of abortion in the mare.71 Ultrasonography made the early detection of multiple conceptus vesicles possible, and it soon became clear that diagnosis of twins before conceptus fixation on day 16-17 after ovulation was beneficial because, even if the conceptuses were adjacent at the time of examination, they could be gently massaged apart or allowed to separate spontaneously (e.g., by returning the mare to a stable and reexamining her 30–60 minutes later) and thereby allow crushing of one of the vesicles between the fingers and the palm of the hand or between the ultrasound probe and pelvic brim, while leaving the second vesicle intact (Fig. 19-7, A-C). In experienced hands, success rates for the manual reduction of a twin to a singleton pregnancy between days 14 and 16 after ovulation are high (>90%72–74), whereas spontaneous reduction to a singleton during the mobile phase is rare and only likely if one of the conceptuses is abnormal.75 After day 17, separation or preferential crushing of one of two unilaterally fixed vesicles without accidentally disrupting both becomes increasingly difficult (Fig. 19-7, D), and the success rate drops accordingly.76
EARLY INTRAUTERINE DEVELOPMENT: THE POST-FIXATION PERIOD
Following fixation, conceptus expansion slows and the vesicle finally loses its perfectly spherical shape, becoming first “guitar-pick”–shaped and then irregular in cross-section.45 It is probably no coincidence that this change in form is associated with cessation in growth and then attenuation of the blastocyst capsule, which eventually disappears completely at around day 23 of gestation.34 Although the rate of increase in conceptus diameter during days 16–35 may be modest when compared with early expansion, the increase in complexity is anything but. In particular, the mesoderm that first appeared at around day 12 continues to expand and differentiate within the so-called “trilaminar omphalopleure” and gives rise to the first primitive blood vessels—the vitelline artery and sinus terminalis—to aid the transport and distribution of nutrients, respiratory gases, and waste products. The embryo proper also undergoes a period of rapid development that includes organogenesis and is exemplified by the formation of a primitive heart and blood vessels (Fig. 19-8) and the development of the allantois as a small sac-like diverticulum protruding from the hindgut. By day 23, the extraembryonic mesoderm covers most of the conceptus, leaving only a small area of “bilaminar omphalopleure” at the abembryonic pole; furthermore, all the major internal organs are discernible in the embryo, the limb buds are beginning to develop, and the allantois has greatly enlarged.77 Two days later the vascularized allantois fuses with the outermost chorion to form the chorioallantois, the future fetal component of the true placenta. At the point where the enlarging allantois meets the by-now regressing yolk sac, a discrete annulate band of chorion develops that is not underlain by vascularized mesoderm; the specialized trophoblast cells within this band proliferate rapidly during days 25–35 of gestation and accumulate in simple folds in the distinctive pale band known as the “chorionic girdle.”78,79 During this same period, the chorioallantois enlarges progressively and the yolk sac shrinks such that, by day 35, the allantois accounts for about 75% of the cross-section of the roughly spherical conceptus.
The Endometrial Cup Reaction and Formation of the True Placenta
The endometrial cup reaction and onset of placentation were comprehensively reviewed by Allen.47 At around day 36, the entire chorionic girdle detaches from the conceptus and adheres to the overlying endometrium, enabling its cells to actively invade the maternal tissue and initiate the process of endometrial cup development.79 During invasion, the girdle cells push through or between the luminal and glandular epithelial cells, penetrate the underlying basement membrane and migrate into the endometrial stroma where they abruptly stop moving, enlarge, and become tightly packed together to form the temporary endocrine organs known as the “endometrial cups.”79,80 The endometrial cup cells secrete the peptide hormone, equine chorionic gonadotrophin (eCG), into nearby lymph sinuses from which it passes into the systemic circulation and stimulates, as a result of its predominantly LH-like biological activity, continued progesterone secretion by the primary CL and the ovulation, or luteinization without ovulation, of any large follicles developing on the ovaries as a result of the continued waves of pituitary FSH secretion.81 This leads to a series of rise(s) in the circulating progesterone concentrations in the dam, which ensure that ovarian progesterone secretion remains elevated until the chorioallantoic placenta is sufficiently well developed to assume the role of providing the progestagens necessary to maintain pregnancy, which occurs somewhere between days 70 and 100 of gestation.82 The endometrium cups persist until days 100–120 of gestation, when, in most mares, they are sloughed as a result of an increasingly intense, maternal, cell-mediated immune response to what are, in essence, immunologically foreign cells.83
Although the endometrial cups are themselves not ultrasonographically visible, their effects usually are; the “secondary” or “accessory” corpora lutea that form as a result of eCG can be seen as multiple CL-like or anovulatory hemorrhagic follicle-like structures on the ovaries (Fig. 19-9). Because it is necessarily associated with pregnancy, elevated blood eCG concentrations between days 45 and 120 of pregnancy have been described as a test to confirm pregnancy.84 However, although eCG analysis is still occasionally used as an indicator of pregnancy in miniature breeds85 or horses that, for other reasons, cannot be examined per rectum, it should be noted that the size and duration of eCG secretion varies considerably between mares (e.g., is considerably lower in the case of donkey or mule pregnancies86), and that pregnancies can develop normally in the absence of endometrial cups, eCG, and secondary CLs.87 In addition, pregnancy loss after the formation of the endometrial cups is not accompanied by loss of the cups (Fig. 19-10).84,88 The cups persist until the normal 100–120 days and, in some cases, much longer.89 Of course, persistence of the endometrial cups after pregnancy loss can lead to a false-positive pregnancy diagnosis when an eCG assay is used and can also make attempts to rebreed mares that suffer from pregnancy loss after day 35 frustrating since many of the developing follicles luteinize instead of ovulating normally.