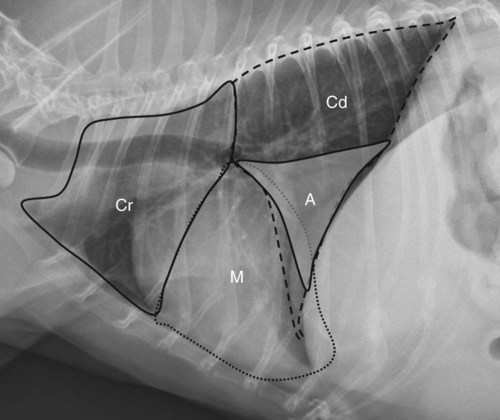
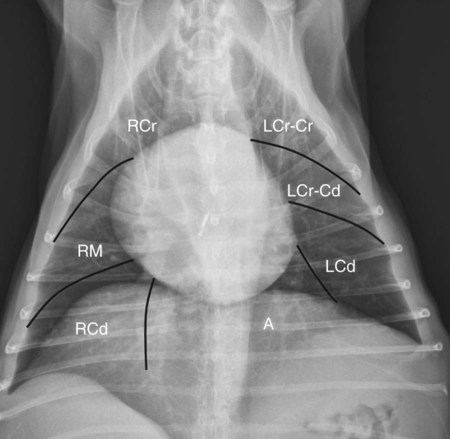
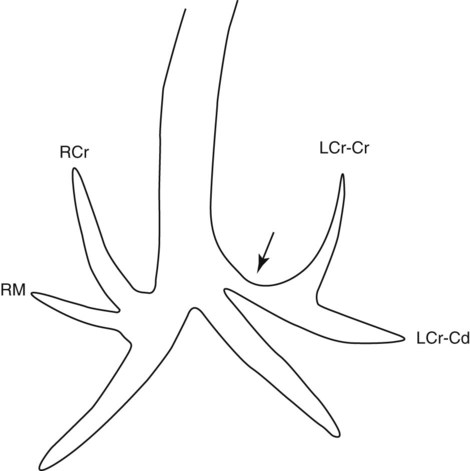
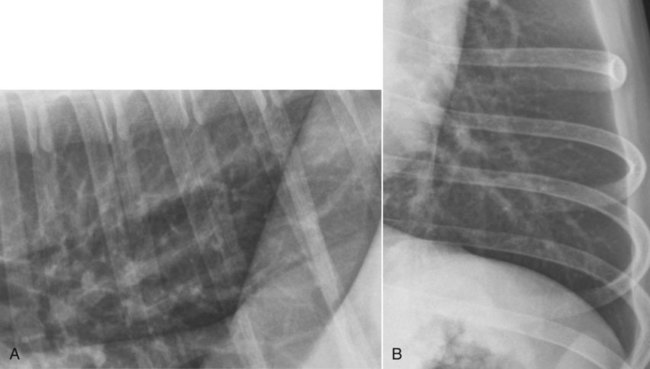
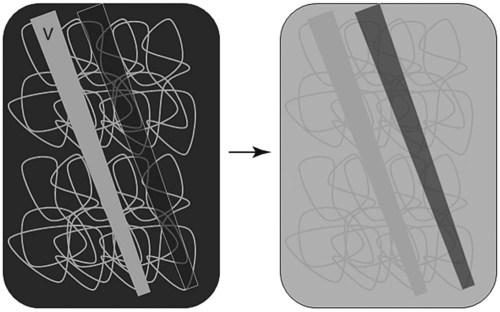
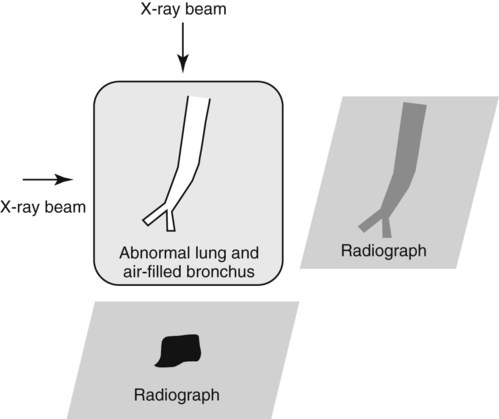
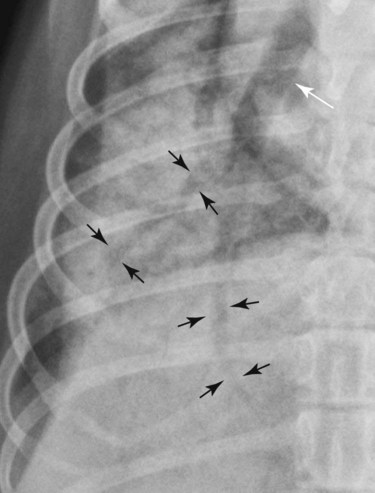
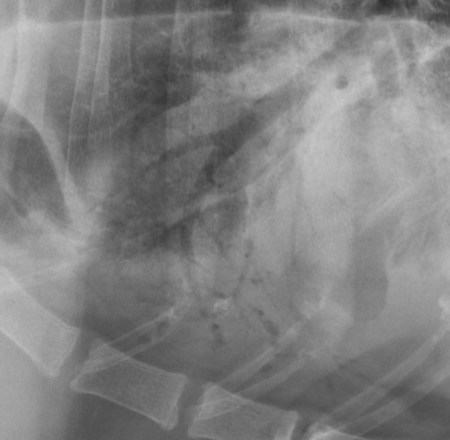
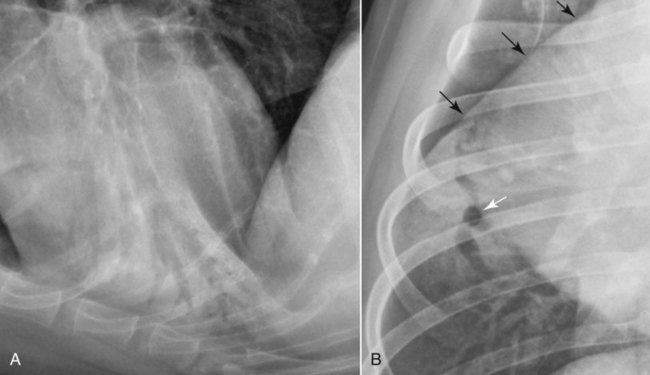
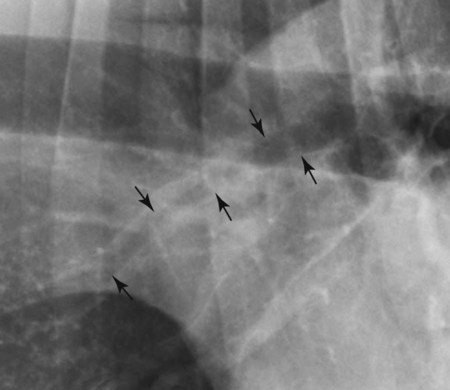
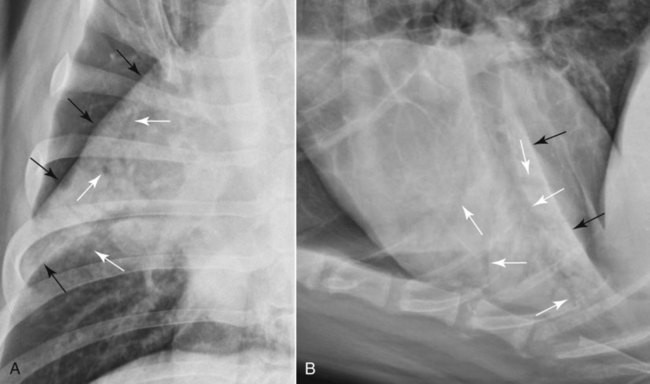
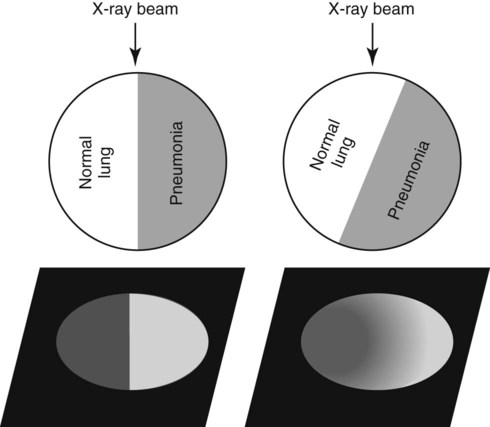
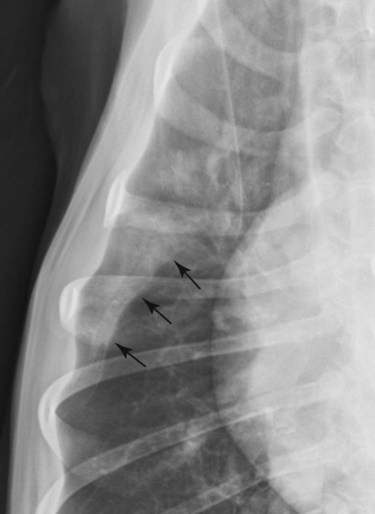
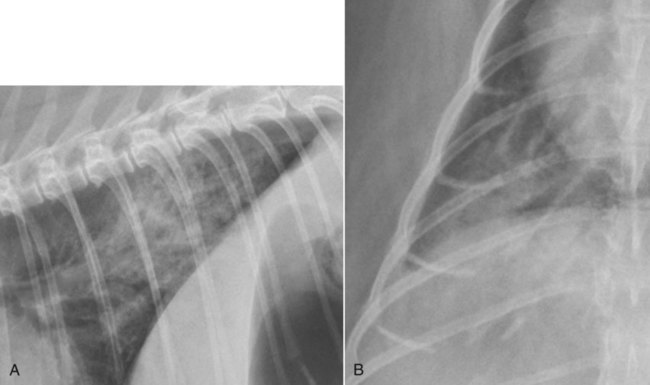
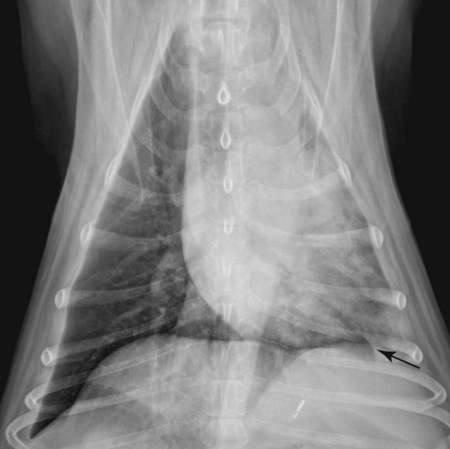
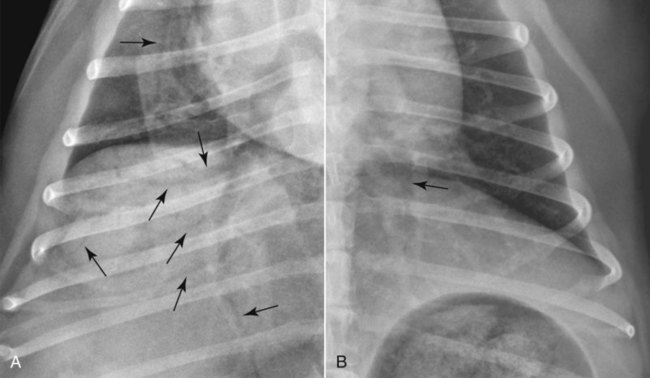
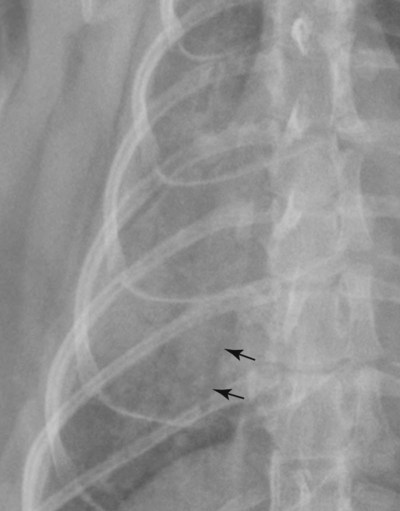
Canine and feline lungs have identical lobation with four lobes of the right lung (the cranial, middle, caudal, and accessory lobes) and two lobes of the left lung (the cranial and caudal lobes). The left cranial lobe is characterized by two distinct segments, the cranial and caudal segments (Figs. 33-1 and 33-2). The location of the lung lobes in Figures 33-1 and 33-2 is approximate because there is considerable overlap of individual lobes in three-dimensional space, and accurate depiction of their exact location in a two-dimensional image is not possible. The cranial and caudal segments of the left cranial lung lobe are not considered individual lobes because their bronchi are not primary branches from the left principal bronchus. Rather, the bronchi of the cranial and caudal segments are themselves branches from a common cranial lobe bronchus that arises from the left principal bronchus. This is different from the right side, where the right cranial and middle lobe bronchi each arise nearly directly from the right principal bronchus (Fig. 33-3). The segments of the left cranial lobe, although considered part of a single lobe, can behave as functionally separate compartments as conditions, such as pneumonia, neoplasia, or hemorrhage, can be localized within only one of the segments. The structure of the lung is also very heterogeneous, providing a complex background upon which changes created by disease occur. Although careful attention to technical detail is important when radiographing all body parts, the complexity of the thorax makes this even more critical. Additionally, there are a large number of patient factors that can influence the radiographic appearance of the lung. These were covered in detail in Chapter 25 and include (1) radiographic technique, encompassing exposure factors and type of acquisition hardware; (2) the effect of the position of the patient on the radiographic appearance of the thorax; (3) effects of recumbent-atelectasis and respiratory phase on radiographic appearance of the lung; and (4) the patient’s habitus. These factors will not be discussed again here, but it is important that they be at the forefront of the thought process when thoracic radiographs are assessed, especially for pulmonary disease. The structure of the lung is somewhat analogous to that of a sponge. There are numerous air spaces, the alveoli, distributed in a fine network throughout a supporting framework of interstitial connective tissue. The interstitium is the infrastructure for distribution of blood vessels, lymphatics, and bronchi throughout the lung. Vessels and bronchi situated near the hilus are relatively large compared with their size at the level of the alveoli. Thus, in a radiograph most opacity caused by normal structures will be created by x-ray absorption in medium to large vessels and bronchi, but the summed, or combined, absorption of x-rays by smaller, individually indistinguishable, vessels and bronchi also contributes to the normal background opacity of the lung. The end result of this is a heterogeneous network of opacities created by the numerous small airspaces, vessels, and bronchi within the framework of the lung (Fig. 33-4). The radiographic appearance of this normal heterogeneous lung will be affected by the factors discussed in Chapter 25, and mentioned again here, creating many opportunities for misinterpretation. Pulmonary disease will also alter this inherent opacity. Therefore, understanding the range of the normal radiographic appearance of lung, and how such is altered by the radiographic technique and patient variability, is critical to being able to detect and categorize lung disease accurately. In this chapter, only disease that results in obvious alteration in pulmonary opacity will be discussed. Radiologists themselves argue about the presence and/or significance of borderline pulmonary changes. The diagnosis of “interstitial disease consistent with the age of the patient” in old dogs is a perfect example. There is no doubt that ageing results in changes in the lungs of dogs that can be detected radiographically. A radiographic pattern consisting of pleural thickening and an increase in nonvascular linear pulmonary markings occurs regularly in older dogs without clinical evidence of pulmonary or cardiovascular disease. These dogs have foci of interstitial fibrosis, often associated with focal areas of emphysema.1 However, sorting out real ageing changes from alterations in pulmonary opacity caused by technical factors or patient variation is difficult, if not impossible, especially for nonspecialists. Therefore, only obvious examples of pulmonary disease are discussed herein. Fortunately, the influx of digital radiography into veterinary medicine facilitates obtaining a specialist opinion on borderline or questionable changes. In the pattern recognition paradigm, the radiographic abnormalities in the lung are categorized in terms of whether they involve primarily the alveoli, the bronchi, or the interstitium. A vascular pattern has also been proposed and could be included in a comprehensive discussion of pulmonary patterns, but in this book the vascular pattern is covered in Chapter 32 and not included here in the discussion of pulmonary patterns. The pattern recognition paradigm has been in use for decades.2–4 The intent is that focusing on the compartment of the lung that is abnormal—that is, the pattern—leads to an organized approach to image evaluation. Also, certain patterns are associated with certain diseases, and these associations streamline the formulation of differential diagnoses for a particular radiographic presentation. The association of a particular pattern or combination of patterns with a list of possibilities has been popularized under the heading of the gamut approach, where the gamut is the list of possibilities.5 Of course, we all use the gamut approach regardless of whether we call it that specifically. The availability of a reference list of exclusions for a roentgen sign just increases the chance of considering all possible causes. If one has never heard of a disease, it cannot be diagnosed. In this chapter, tables of considerations are given for certain roentgen signs, but these are not intended to be comprehensive, because the emphasis is on major categories of disease seen commonly in private practice. Some discussion of the word infiltrate is necessary when talking about lung patterns. Infiltrate is used occasionally to describe an abnormal pulmonary pattern when the specific cause is not known, which it rarely is. For example, one might conclude that there is an interstitial infiltrate in the left caudal lobe. Inherent in this usage, by some, is the implication that the disease is spreading through the organ without disturbing the normal architecture, but for others it simply means the presence of an abnormal substance in the lung. A few years ago a survey was performed where physician radiologists were asked to interpret the word infiltrate when used in a thoracic radiographic report and comment if the word was helpful in clinical management of patients.6 Nearly 90% replied that infiltrate implied more than one pathophysiologic condition, slightly more than half thought infiltrate could mean any of six or more different pathophysiologic conditions, and only about one third thought the term was helpful in patient care. Thus, the conclusion was that infiltrate was nonspecific and imprecise and did not usually enhance patient care. This report generated numerous responses from other radiologists, many of whom disagreed with the conclusions.7,8 Even before the survey, the legitimacy of infiltrate as a radiologic descriptor had been questioned, and some had concluded that “. . . there just doesn’t appear to be any better way of expressing the concept of extension or expansion without associated anatomic distortion than the word infiltrate.”9 So, in the discussion of lung patterns in this book, infiltrate is avoided whenever possible for the sake of reducing confusion. Almost always, the word pattern can be substituted. However, there are those certain circumstances, such as a tumor winding its way through the interstitium, where infiltrate seems to capture the process, and if infiltrate is used in this chapter, that concept is inherent. Air bronchograms are considered the hallmark sign of an alveolar pattern. The air bronchogram sign was named by Felson10 but was first described in principle by Fleischner in 1948.11 An air bronchogram is defined as an air-filled bronchus traversing a region of abnormal lung where alveolar air has been replaced by exudate, hemorrhage, or edema fluid. Critical requisites for air bronchogram visualization are (1) air within bronchi has not been replaced by cells or fluid, and (2) the extent of air replacement in the alveoli has been extensive enough to provide adequate background opacity—in other words, enough x-ray absorption—for the air-containing bronchi to be seen. Under these circumstances, the air-filled bronchi then appear radiolucent against the increased opacity of the abnormally opacified lung (Fig. 33-5). Typically, air bronchograms appear as a tubular radiolucent structure with occasional branching. But, if the air-filled bronchus is aligned with the primary x-ray beam such that it is struck end-on rather than side-on, it will appear as a circular radiolucency instead of being tubular (Fig. 33-6). The actual appearance of an air bronchogram in a radiograph depends on how much alveolar air has been replaced by fluid or cells; the extent of the distribution of the alveolar pattern; and, as already noted, the geometric relationship of the air-filled bronchus with the primary x-ray beam (Figs. 33-7 through 33-9). Air bronchograms are particularly valuable for diagnosing an alveolar pattern in instances where the absolute intensity of the alveolar disease is borderline, making the opacity change itself difficult to recognize if the bronchial lumen conspicuity was not increased (Fig. 33-10). The radiographic appearance of an air bronchogram is relatively standard, and they are easy to recognize, as long as the underlying principles are understood. A common mistake is to interpret the radiolucent region between a pulmonary artery–vein pair as an air bronchogram (Fig. 33-11; see Fig. 33-10). The well-defined margin of the increased opacity next to the bronchial lumen in the instance of a bronchus being positioned between two vessels is not typical of the more diffuse nature of the increased opacity created by an alveolar pattern that surrounds a bronchus and creates an air bronchogram. A lobar sign refers to the sharp margin created when a lobe with increased opacity abuts a normally aerated lobe that has less opacity (Fig. 33-12; see Fig. 33-9, B). Usually, a lobar sign is observed when a lobe having increased opacity caused by alveolar air being replaced with fluid, exudate, or hemorrhage abuts a normally aerated lobe. Occasionally, a lung mass will extend to the periphery of a lobe, and this too can create a lobar sign with the adjacent normal lobe, although in this instance, the shape of the lobar sign will be altered by the mass and appear differently, more round for example, compared with the expected shape of a normal, slightly curving, interlobar junction. To correctly identify a lobar sign, it is necessary to know normal lung lobe anatomy and where lung borders are located. If a lobar sign is seen in one view, it may not be detected in the orthogonal view because for the lobar sign to be visible the junction between the affected lobe and the adjacent normal lobe must be struck tangentially (i.e., in a parallel fashion) by the x-ray beam (Fig. 33-13). If the junction is struck at an angle, the lobar sign will not be seen. A lobar sign can be the only indicator of an alveolar pattern, especially if the extent of the disease is limited (Fig. 33-14). Although air bronchograms and lobar signs are common indications of an alveolar pattern, sometimes neither will be seen. Air bronchograms may not be seen if the alveolar disease is not concentrated adequately around a bronchus for the bronchial lumen to become visible. This might occur if sufficient contrasting alveolar material is not present or the disease has also resulted in air displacement from the bronchi. A lobar sign will not be seen if the alveolar disease does not extend to the periphery of a lobe, if adjoining lobes are both affected to the same extent, or if the lobe junction is not struck parallel by the x-ray beam. Unfortunately, these scenarios are common, and in many animals with an alveolar pattern, neither an air bronchogram nor a lobar sign will be present. In these patients, the diagnosis of an alveolar pattern is based on the finding of a region of lung opacification that is too intense per unit area to be caused by disease confined to the bronchial tree or unstructured disease confined to the interstitium. At the same time, this region of intense lung disease does not have the sharp margins expected of a lung mass. Thus, an alveolar pattern is sometimes diagnosed by exclusion (i.e., the lung disease is too intense to be caused by a bronchial or unstructured interstitial pattern), and it also does not have the margin characteristics of a lung mass (Figs. 33-15 and 33-16). As noted before, an alveolar pattern results from the presence of cells or fluid in the alveolar spaces. Common causes of an alveolar pattern and generalizations for the distribution of the disease within the lungs are given in Table 33-1. Table • 33-1 The association between atelectasis and an alveolar pattern deserves special mention. Up to this point the discussion of an alveolar pattern has focused on conditions where alveolar air is replaced by another substance. However, alveolar air can be diminished simply by the lung becoming collapsed, either from extrinsic compression, bronchial obstruction, or reduced ventilation. These situations will also result in an increase in lung opacity and, if the atelectasis is severe enough, the visualization of an alveolar pattern. A key component of atelectasis is a mediastinal shift. Therefore, finding mediastinal displacement toward the direction of an alveolar pattern is evidence that at least a portion of the alveolar pattern results from atelectasis. The determination whether all of the pulmonary opacification is caused by atelectasis alone or a combination of atelectasis and alveolar disease cannot be made from radiographs. Occasionally, the clinical history will be helpful in making this distinction, but lung sampling may be necessary to obtain the definitive answer (Figs. 33-17 and 33-18).
The Canine and Feline Lung
Pulmonary Anatomy
Radiographic Appearance of Normal Lung
Paradigms for Assessing Pulmonary Disease
Pattern Recognition Paradigm
Alveolar Pattern
CAUSE
DISTRIBUTION*
PREVALENCE
Pneumonia
Ventral
Common
Cardiogenic pulmonary edema
Variable
Common
Noncardiogenic pulmonary edema
Dorsocaudal
Less common
Hemorrhage
Trauma
Variable
Common
Coagulopathy
Variable
Less common
Thromboembolism
Variable
Less common
Atelectasis
Variable
Common
Allergy (eosinophilic)
Variable
Rare
Primary lung tumor
Variable
Rare

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


The Canine and Feline Lung