CHAPTER 27 The Bone Marrow
INDICATIONS
The most common indication for bone marrow cytologic examination is recognition of hematologic abnormalities not readily explained by a good history, physical examination, chemistry profile, and/or other clinical procedures. Bone marrow examination is often rewarding in evaluating animals with nonregenerative anemia, persistent neutropenia, or persistent thrombocytopenia.1–3
Bone marrow cytologic evaluation can also be used to assess the marrow’s involvement in some neoplastic conditions, such as lymphoid neoplasia and mast cell neoplasia; to identify suspected infectious agents, such as Histoplasma capsulatum, Leishmania donovani, Ehrlichia spp., Cytauxzoon felis, and Toxoplasma gondii; and to evaluate patients with evidence of hyperglobulinemic conditions (e.g., multiple myeloma, lymphoma, ehrlichiosis/anaplasmosis, leishmaniasis, and disseminated histoplasmosis) (Box 27-1).4–8
Box 27-1 Bone Marrow Cytologic Evaluation
Historical or Physical Findings
Abnormal CBC Findings
CBC, Complete blood count; WBC, white blood cell; RBC, red blood cell
CONTRAINDICATIONS
Contraindications of bone marrow biopsies are few, and complications are uncommon. Restraint, sedation, and anesthesia usually pose a greater risk to the patient than the biopsy procedure. Bone marrow aspiration biopsies can often be collected without sedation; general anesthesia is seldom necessary. Hemorrhage is a theoretical concern in thrombocytopenic animals, but significant bleeding rarely occurs, even in severely thrombocytopenic animals. Bleeding complications can be controlled with fresh whole blood or appropriate component therapy.4,9,10 Iatrogenic marrow infection is possible, but the risk is miniscule, especially if the area of skin through which the aspirate is collected has been properly prepared. The main contraindication for bone marrow biopsy is performing an unnecessary biopsy. Evaluation of a current blood smear and a thorough clinical workup are mandatory before performing a bone marrow biopsy.
SAMPLE COLLECTION AND PREPARATION
Proper collection and preparation of marrow are necessary for maximal diagnostic usefulness.4,5,7,11–13 Bone marrow degenerates rapidly after collection or the animal’s death; therefore bone marrow samples from dead animals should be collected immediately (within 30 minutes) after the animal’s death. Regardless of whether the animal is alive or dead, bone marrow cytologic preparations should be prepared immediately after collection. Interestingly, granulocytes appear to be the first cells to undergo significant morphologic distortion after marrow sample collection or the animal’s death. Granulocytic nuclei swell and, losing their contorted lobulated pattern, become large and round to ovoid. The nuclear chromatin pattern loses its dense clumped areas and stains less intensely and more uniformly. As a result, neutrophils that have undergone this alteration may be mistaken for blast cells. This error can lead to misdiagnosis of neoplasia. Extreme caution must be used when examining samples from animals dead for 30 minutes or more before slide preparation. These samples can be examined for cellularity, organisms, mast cell infiltration, erythrophagocytosis, plasmacytosis, and accumulation of iron-containing pigments, but examination for evidence of neoplasia can result in misdiagnosis. The same requirements and limitations apply for bone marrow samples submitted for histopathologic examination.
Aspiration Biopsy
Site
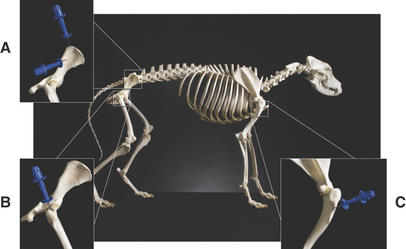
Bone marrow aspiration biopsies may be collected from the proximal humerus, iliac crest, trochanteric fossa of the femur, and sternebrae of dogs and cats (Figure 27-1).4,5,7,11–13 The proximal humerus and iliac crest in large dogs and the proximal humerus and trochanteric fossa in small dogs and cats are the most accessible. As a result, they are the most commonly used sites. Because of the danger of penetrating the thoracic cavity, the sternebrae should be avoided in cats and small dogs. For the same reason, the ribs should be used only when incisional biopsies are performed. The trochanteric fossa may not be approachable in large, well-muscled, or very obese patients. Also, the cortical bone of the trochanteric fossa may be so dense in older dogs that it prevents easy penetration of the marrow cavity. Because of the small diameter of the bones of cats and small dogs, the trochanteric fossa often supersedes the iliac crest as a site for bone marrow aspiration in these animals.
Smear Preparation without EDTA
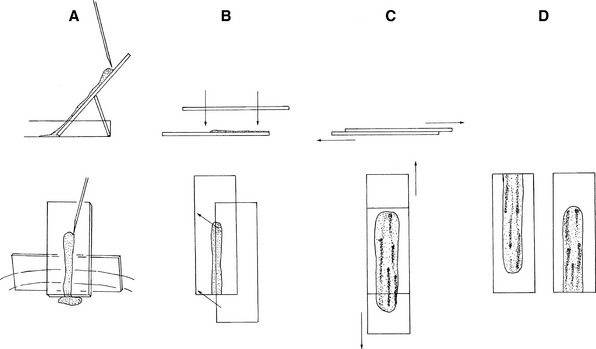
Then the sample is immediately expelled directly onto a glass microscope slide. Direct smears similar to blood smears can be made and squash preps should also be prepared by tilting a slide 45 to 70 degrees, allowing the blood to drain from the slide into a watch glass or Petri dish (Figure 27-2). Marrow flecks tend to adhere to the glass microscope slide. A second glass microscope slide is placed perpendicularly across the marrow flecks adhered to the first slide, causing the marrow flecks to spread. The two slides are then smoothly pulled apart in a horizontal plane, dispersing the flecks.
Smear Preparation with EDTA
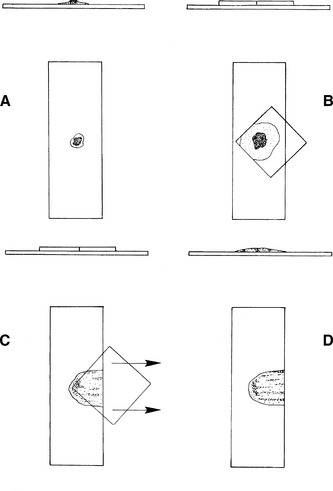
After the fleck is transferred to the glass microscope slide, a 22- × 22-mm coverslip is placed over the fleck at a 45-degree angle to the glass microscope slide, allowing one corner of the coverslip to hang over the edge of the microscope slide (Figure 27-3). When the coverslip is placed over the fleck, the fleck spreads to about twice its previous size. Some smears should be made without any pressure other than that caused by the coverslip, and others should be made with gentle thumb pressure sufficient to cause the smears to spread to about twice the diameter caused by coverslip pressure alone. This ensures that some of the flecks are optimally spread.
Core Biopsy
Core biopsies can be collected with a Jamshidi infant (cats and small dogs) or pediatric (large dogs) marrow biopsy needle. The same procedure is used for collecting core biopsies as described for collecting marrow aspirate biopsies, except after the point of the needle has penetrated the cortex of the bone and entered the marrow cavity, the stylet is removed from the needle and the needle is advanced about 3 mm with a rotating motion.4,5,7,8,11–13 This cuts and collects a core of bone marrow. The needle is removed from the animal, and the core of marrow is forced onto a glass microscope slide by passing the stylet through the barrel of the needle from the hub end or retrograde from the needle end if the biopsy needle is tapered.
COLLECTING MARROW SAMPLES FROM DEAD ANIMALS
Although samples can be collected from most flat bones and the extremities of most long bones of dead animals, it is advisable to collect marrow samples from the same location(s) used in live animals (see Figure 27-1). Remember that in adults, the central areas of long bones contain mostly fat. Access to the marrow can be gained by sawing the bone, cutting it with rongeurs, or fracturing it. If the bone is sawed, heat generated at the saw line can damage adjacent cells; therefore samples from sawed bones should be collected well away from the saw line. Marrow is dug from between bony trabeculae, trying to avoid collecting bone spicules. The marrow is placed on one end of a glass microscope slide and gently rolled (by lifting upward with a needle or other instrument at the back of the sample) the length of the slide. Several slides are prepared from samples collected from several different areas of the bone marrow. The slides are air-dried and stained as described later.
CELLS IN MARROW
To evaluate cytologic preparations of bone marrow, one must be able to recognize the normal cells that occur in bone marrow, the neoplastic cells that have a propensity for infiltrating bone marrow, the organisms that can infect the marrow, and the processes (e.g., erythrophagia) that occur in some pathologic conditions.4–9,13,14
Erythroid Series
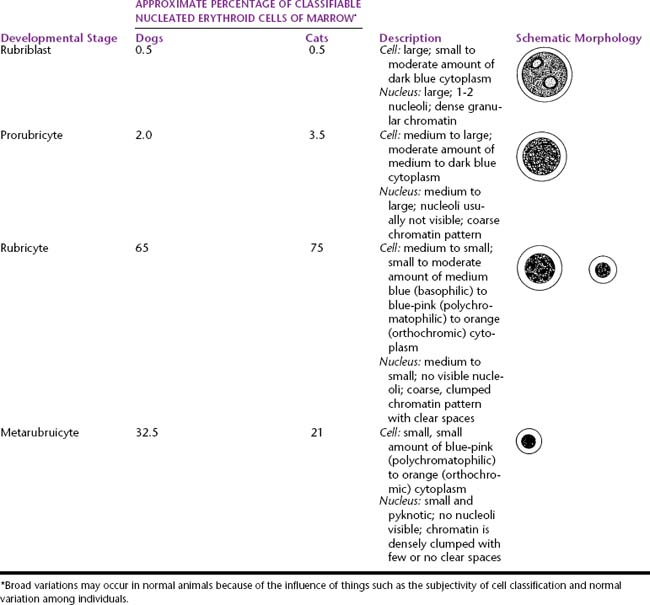
During proliferation and maturation, erythroid progenitor cells undergo 4 to 5 mitoses, producing 16 to 32 daughter cells. As they mature, erythroid cells decrease in size, their nuclei condense, and their cytoplasm changes from dark blue to red-orange. The general characteristics of the different cell stages of erythroid production are described later. Table 27-1 lists the different stages of erythroid development and provides an estimate of the relative proportions and a brief description and a generalized schematic of the classic morphology of each stage.
Table 27-1 Nucleated Erythroid Cell Developmental Stages and Their Relative Percentage, Description, and Schematic Morphology
Rubriblasts
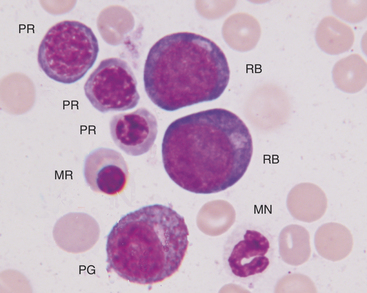
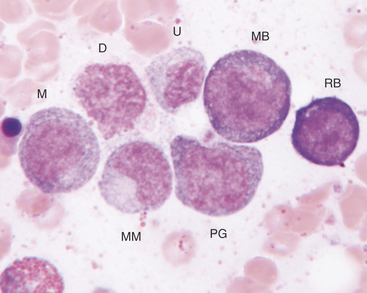
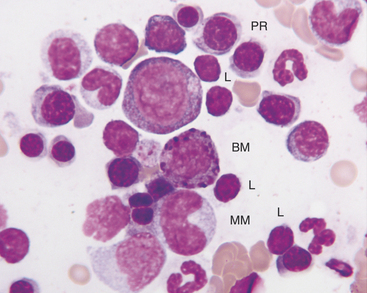
The rubriblast is the most immature identifiable erythroid cell. Its nucleus is round with a smooth nuclear border, a fine granular chromatin pattern, and one or two pale to medium blue nucleoli (Figures 27-4 and 27-5). Its cytoplasm is intensely basophilic and forms a narrow rim around the nucleus. The rubriblast has the highest nuclear-to-cytoplasmic ratio of the erythroid series.
Rubricytes
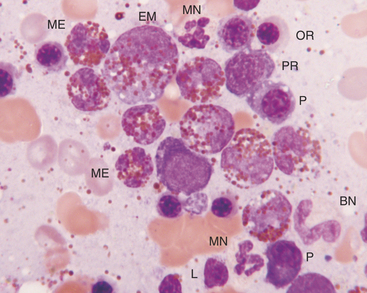
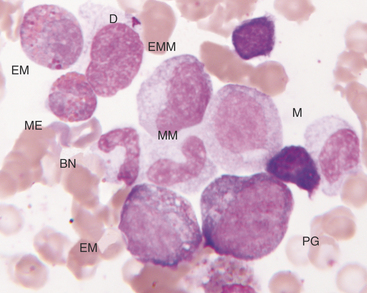
The next stage of erythroid maturation is the rubricyte. This stage is divided into basophilic and polychromatophilic rubricytes or sometimes divided into basophilic, polychromatophilic, and orthochromic rubricytes. The rubricyte and its nucleus are smaller than the prorubricyte and its nucleus. The rubricyte nucleus has an extremely coarse chromatin pattern that may resemble the spokes of a wheel. Cytoplasm is blue (basophilic) to bluish-red-orange (polychromic or polychromatophilic) to red-orange (orthochromic) (Figures 27-6 and 27-7; see Figure 27-4). The nuclear-to-cytoplasmic ratio is less than that of prorubricytes but greater than that of metarubricytes (the next stage of maturation). Mitosis occurs in the early rubricyte stage but ceases by the later rubricyte stages.
Metarubricytes
The next stage of erythroid development is the metarubricyte. Its nucleus is extremely pyknotic and appears black, without a distinguishable chromatin pattern (see Figure 27-4). Cytoplasm may be polychromatophilic or orthochromic.
Granulocyte Series
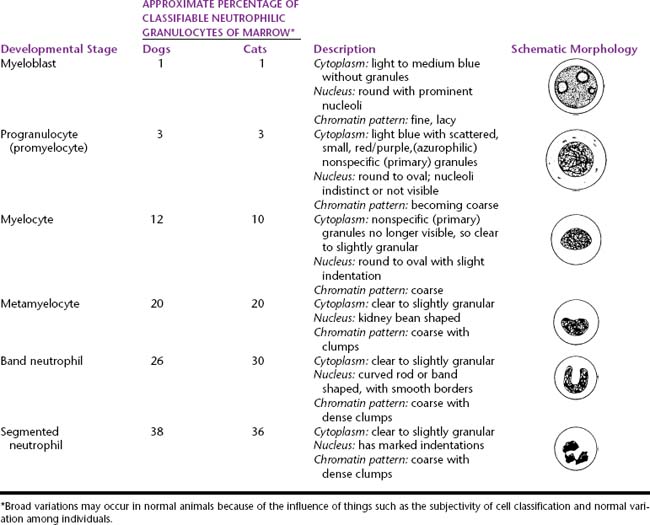
Myeloid progenitor cells usually undergo four mitoses; however, depending on circumstances, a mitosis may be skipped or additional mitoses may occur. The developmental stages, immature to mature, of the granulocyte series are myeloblast, progranulocyte (promyelocyte), myelocyte, metamyelocyte, band cell, and segmented granulocyte (mature neutrophil, eosinophil, and basophil). The myeloblast, myelocyte, and metamyelocyte stages of neutrophilic granulocytes cannot be reliably differentiated from monocytes. Table 27-2 lists the different stages of development of the granulocyte series and gives the relative proportions and a brief description of the classic morphology of each stage.
Table 27-2 Developmental Stages of Neutrophils and Their Relative Percentage, Description, and Schematic Morphology
Myeloblasts
The myeloblast is large and may be round or irregular in shape. It has a large nucleus with a fine to finely stippled chromatin pattern and one or more visible nucleoli (see Figure 27-5). The cytoplasm is blue to blue-gray and does not contain visible granules. The myeloblast has the highest nuclear-to-cytoplasmic ratio of any of the granulocytic developmental stages.
Progranulocytes (Promyelocytes)
The next stage of granulocyte development is the progranulocyte or promyelocyte. Often, the progranulocyte is slightly larger than the myeloblast due to increased cytoplasm. The major differentiating feature of the progranulocyte is the presence of scattered, small, azurophilic (red-purple) primary granules throughout the cytoplasm (Figure 27-8; see Figure 27-5). Primary granules are also called nonspecific granules. Nuclei are the same size to slightly smaller than nuclei of myeloblasts, have lacy to coarse chromatin patterns, and may contain visible nucleoli (which are usually fewer and less prominent than those of myeloblasts) or nucleolar rings. Nucleolar rings are rings of densely staining chromatin that demark obscured nucleoli. The nuclear-to-cytoplasmic ratio of promyelocytes is less than that of myeloblasts. The presence of primary granules allows progranulocytes to be recognized as members of the granulocyte series and differentiates them from the monocyte series. Therefore progranulocytes can be identified more specifically than myeloblasts, neutrophil myelocytes, and neutrophil metamyelocytes, which lack visible primary granules. As a result, the term progranulocyte, instead of promyelocyte, can be used to identify members of this developmental stage.
| Hypocellular marrow | Normocellular Marrow | Hypercellular Marrow |
|---|---|---|
| FeLV suppression (cat)–A | FeLV suppression (cat)–A, d | Histoplasmosis–A |
| Ehrlichiosis (dog)–A | Ehrlichiosis (dog)–A, d | Ehrlichiosis (dog)–TP, e |
| Renal insufficiency–NA | Histoplasmosis–A, d | Myeloproliferative disease–A |
| Marrow necrosis–A, a | Myeloproliferative disease–A, d | Lymphosarcoma–A |
| Radiation, cytotoxic drugs, etc–A, a | Lymphosarcoma–A, d | Anemia of inflammatory disease (i.e., anemia of chronic disease)–NA |
| Myelofibrosis (sclerosis)–A, b | ||
| Pure red cell aplasia–NA, c | ||
| Immune-mediated idiopatic |
FeLV, Feline leukemia virus; A, one to all of the marrow cell lines may be affected; NA, nonregenerative anemia; NP, neutropenia; TP, thrombocytopenia; a, anemia may not be present in the acute phase; b, flecks, if any are collected, may appear small but hypercellular because fibrous tissue has replaced most of the marrow elements, but a few pockets of hypercellular marrow may remain; c, rarely, thrombocytopenia and/or neutropenia may also be present; d, usually associated with hypocellular marrow; e, ehrlichiosis can be associated with hypocellular marrow, but then it is usually in the acute to subacute phase of infection and is associated with thrombocytopenia alone—clinical signs are often unapparent.
Myelocytes
The next developmental stage of the granulocyte series is the myelocyte. The disappearance of the primary granules (seen in the progranulocyte stage) aids differentiation of the myelocyte from the progranulocyte. Also, myelocytes are smaller than progranulocytes, and their nuclear chromatin is more dense, giving a coarser pattern (Figure 27-9; see Figures 27-5 to 27-8). Nuclei are round to oval, and nucleoli are not visible. Cytoplasm is light blue to clear and contains secondary (specific) granules, which are poorly visualized in the neutrophil series but give eosinophils and basophils their characteristic appearance.
The granules of feline eosinophils are rod shaped, and the granules of canine eosinophils are pleomorphic (but typically round) and variable in size (see Figures 27-7 and 27-8). Feline basophils contain granules that are oval, tend to stain lavender, and fill the cytoplasm. The oval granules may appear round when viewed end-on. Immature feline basophils contain many red-purple granules (see Figures 27-6 and 27-9). Canine basophils contain a few to many round, variably sized, red-purple (metachromatic) granules. The myelocyte is the last stage capable of mitosis.
Metamyelocytes
The next stage of granulocyte development is the metamyelocyte. Its nucleus is classically described as kidney bean shaped. Generally, cells with nuclei having indentations extending less than 25% of the way into the nucleus are classified as myelocytes, whereas those with nuclei having indentations extending 25% to 75% of the way into the nucleus are classified as metamyelocytes (see Figure 27-6). The cytoplasmic characteristics of metamyelocytes are similar to those of myelocytes, with the neutrophil myelocyte cytoplasm being clear. This stage and subsequent stages do not undergo mitosis.
Band Cells
The next stage of granulocyte development is the band cell. It has a nucleus with a curved band or rod shape and smooth, parallel sides (see Figures 27-7 and 27-8). Some chromatin clumps are present. No area of the nucleus has a diameter less than one-half the diameter of any other area of the nucleus. Membrane irregularity and excessive narrowing of the nucleus warrant classifying of the cell as a mature neutrophil. The cytoplasmic characteristics of band cells are similar to those of metamyelocytes.
Segmented Granulocytes
The final stage of granulocyte development is the segmented granulocyte (mature neutrophil, eosinophil, or basophil). Its nucleus is lobate or has areas of marked constriction (see Figure 27-7). The nuclear border is often irregular with large, dense chromatin clumps. Its cytoplasmic characteristics are similar to those of myelocytes, metamyelocytes, and band cells.
Thrombocyte (Megakaryocyte) Series
Promegakaryocytes
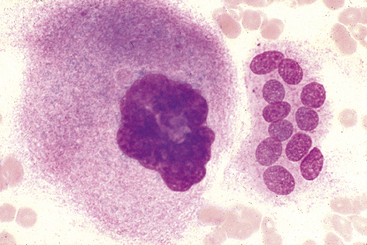
The promegakaryocyte is the next recognizable stage. It has deep blue cytoplasm and contains two to four nuclei that may appear separate but are linked by thin strands of nuclear material (see Figure 27-9). Promegakaryocytes are much larger than WBC and RBC precursors.
Megakaryocytes
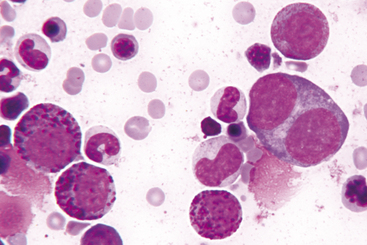
The megakaryocyte is the next developmental stage. Megakaryocytes are gigantic (50 to 200 microns in diameter) and contain more than four nuclei that are joined and form a lobulated mass (Figures 27-10 and 27-11). The larger the cell, the greater the nuclear ploidy (number). Megakaryocytes with deeply basophilic cytoplasm are less mature than those with light blue cytoplasm containing eosinophilic granules. Immature megakaryocytes are sometimes called basophilic megakaryocytes, and mature megakaryocytes are sometimes called eosinophilic or granular megakaryocytes. Bare nuclei of megakaryocytes may be seen in bone marrow cytologic preparations. They may represent nuclei of megakaryocytes that have shed their cytoplasm as platelets into the peripheral blood or megakaryocytes whose cytoplasm has been torn from their nuclei during smear preparation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree