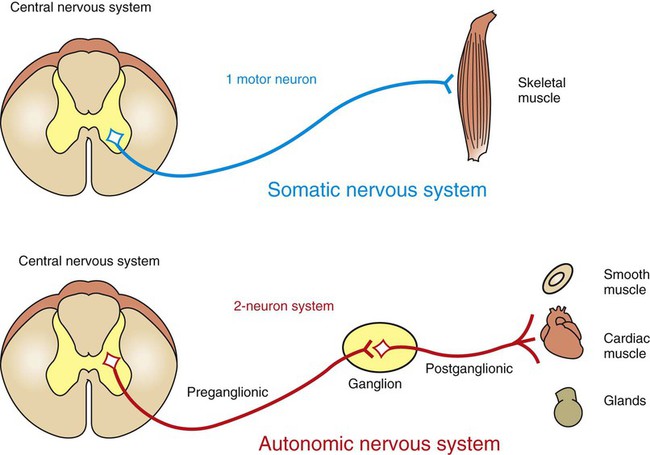
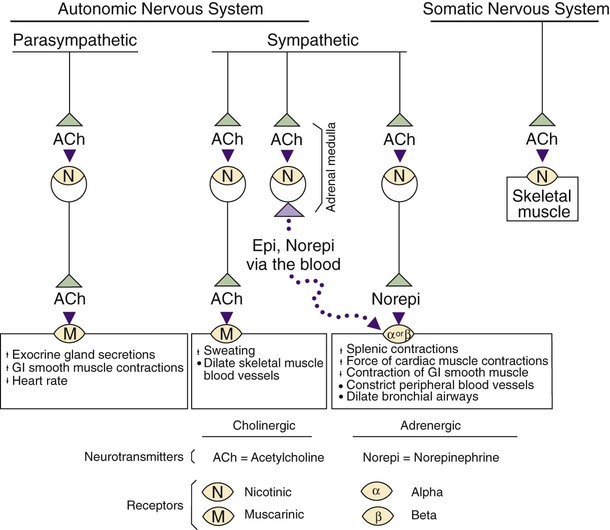
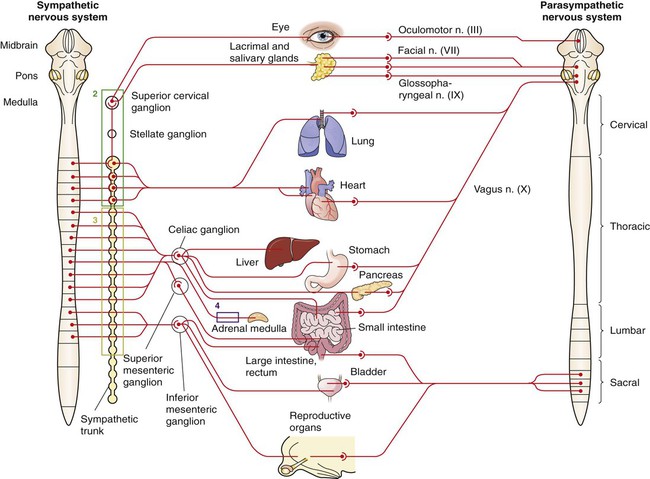
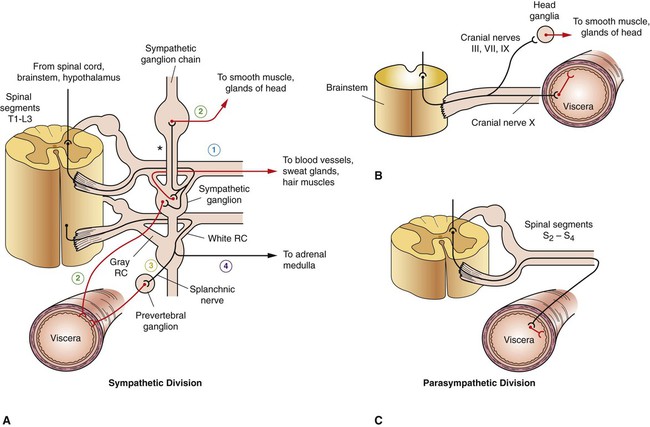
1. The peripheral autonomic nervous system differs from the somatic motor system in a number of important ways. 2. The peripheral autonomic nervous system has two subdivisions that originate in the central nervous system and one that does not. 3. The sympathetic nervous system originates from the thoracolumbar spinal cord. 4. The parasympathetic nervous system arises from the brainstem and sacral spinal cord. 5. Most sympathetic and parasympathetic neurons secrete either acetylcholine or norepinephrine as a neurotransmitter. 6. Acetylcholine and norepinephrine have different postsynaptic receptors. 7. Neurotransmitters other than acetylcholine and norepinephrine play some role in peripheral autonomic function. 8. There are general differences in sympathetic and parasympathetic function. 9. Visceral afferent (sensory) neurons play an important role in autonomic nervous system function. 10. The autonomic nervous system participates in many homeostatic reflexes. 11. Preganglionic neurons are influenced by many regions of the brain. The autonomic nervous system (ANS) is a part of the nervous system that is generally not under conscious, voluntary control, nor is the organism usually conscious of its operation. The ANS is commonly defined as a peripheral motor system innervating smooth muscle, cardiac muscle, glandular tissue and the organs of the body cavity, known as viscera (e.g., stomach, urinary bladder), that these tissues often comprise. It should be kept in mind, however, that these peripheral targets and their motor innervation are usually part of reflex pathways that also include visceral afferents (see Chapter 3) and central nervous system structures (e.g., hypothalamus), both of which are sometimes included in broader definitions of the ANS. The ANS differs from the somatic motor system in its target organs, in the number of neurons in its peripheral circuit, and in the nature of the synapse at the target organ. The somatic motor system innervates skeletal muscle, which is the muscle responsible for all movements of the body, as described in Chapters 5 and 6. In contrast, the ANS innervates smooth muscle, cardiac muscle, and glandular tissue (Figure 13-1). Cardiac muscle is the muscle of the heart (see Chapter 19). Smooth muscle is the muscle in blood vessels, in most of the gastrointestinal tract, in the bladder, and in other hollow visceral structures. Gland cells can also be part of visceral organs, as well as comprising nonvisceral glands (e.g., salivary glands, lacrimal gland). The ANS also differs in the number of neurons it has in the peripheral nervous system (see Figure 13-1). The somatic nervous system has one neuron whose cell body is located in the central nervous system (CNS) and whose axon extends, uninterrupted, to the skeletal muscle, where the peripheral chemical synapse occurs. In contrast, the ANS has two peripheral neurons. The first, called a preganglionic neuron, also has its cell body in the CNS, but its axon innervates a second neuron in the chain, called the postganglionic neuron. The latter’s cell body is in a peripheral structure called a ganglion, a collection of neuronal cell bodies outside the CNS. There are chemically mediated synapses both between the preganglionic and postganglionic neurons and between the postganglionic neuron and the cells of its target organ. The ANS also differs from the somatic motor system in the amount of myelin along the peripheral axons; the autonomic postganglionic neurons usually have slowly conducting, unmyelinated axons. In addition, somatic motor neurons always excite their skeletal muscle targets, whereas the autonomic postganglionic neurons can either excite or inhibit their targets. Furthermore, unlike the narrow synaptic cleft at the focal neuromuscular junction of a skeletal muscle cell, ANS target cells are often activated at a greater distance, by a highly branched postganglionic neuron with synaptic boutons (called varicosities; see Figure 27-7) distributed all along the length of these branches. This can contribute to a longer latency for, and greater spatial distribution of, postsynaptic cell activation by autonomic postganglionic neurons. The peripheral ANS is divided into two major subdivisions based on the respective CNS origin of their preganglionic neurons and on their synaptic transmitters at the target organ. These two subdivisions are the sympathetic nervous system and the parasympathetic nervous system. The enteric nervous system can be considered a third subdivision of the peripheral ANS. It is an extensive network of interconnected sensory, motor and interneurons within the gut (gastrointestinal tract) wall that can control gut function independently of the CNS. However, these neurons can also be influenced by the CNS through input from the sympathetic and parasympathetic subdivisions. The enteric nervous system will be discussed in more detail in reference to the regulation of gastrointestinal function in Chapter 27. The sympathetic nervous system generally has short preganglionic and long postganglionic axons. Preganglionic axons of the sympathetic nervous system leave the spinal cord by way of the ventral roots of the first thoracic through the third or fourth lumbar spinal nerves (Figure 13-2). For this reason, the sympathetic nervous system is often called the thoracolumbar system. The preganglionic axons pass through the ventral root and then a communicating branch (white ramus) to enter the paravertebral sympathetic ganglion chain (also called the sympathetic trunk), where most synapse with a postganglionic neuron (Figure 13-3, A). The ganglion chain actually extends from cervical to sacral regions and some of the thoracolumbar preganglionic neurons extend their axons rostrally or caudally within the chain to reach these cervical and sacral ganglia (see Figure 13-3, A, asterisk). A large complement of postganglionic axons from each of the chain ganglia enter nearby spinal nerves, through a different communicating ramus (gray ramus), and travel to the body wall or extremities to control blood vessels, sweat glands, or hair erector muscles (see Figure 13-3, A, #1). Another complement of these postganglionic neurons, mainly from thoracic or cervical chain ganglia, does not enter spinal nerves but forms separate nerves that travel respectively to thoracic viscera (e.g., heart, bronchi) or to organs and glands of the head (e.g., eye, lacrimal gland; see Figure 13-3, A, #2). Some of the thoracolumbar preganglionic axons simply pass through the sympathetic chain ganglia without synapsing there. These axons form splanchnic nerves that synapse with postganglionic neurons in prevertebral ganglia (see Figure 13-3, A, #3), usually named for neighboring blood vessels (e.g., celiac, mesenteric). Postganglionic neurons of the prevertebral ganglia innervate abdominal and pelvic visceral organs. Some of the aforementioned splanchnic nerve fibers bypass the prevertebral ganglia and continue all the way to the adrenal medulla, where they synapse with rudimentary postganglionic neurons that make up the adrenal medullary secretory cells (see Figure 13-3, A, #4). These vestigial postganglionic neurons secrete their transmitter substance directly into the circulating blood. The transmitter substance, acting as a true hormone, is carried by the blood to all tissues of the body. The parasympathetic nervous system generally has long preganglionic and short postganglionic axons. Preganglionic axons of the parasympathetic system leave the CNS by way of cranial nerves III (oculomotor), VII (facial), IX (glossopharyngeal), and X (vagus) and through several sacral spinal nerves. For this reason, it is called the craniosacral system (see Figure 13-2). The parasympathetic preganglionic axons leaving through cranial nerves III, VII, and IX synapse in well defined ganglia outside the skull (e.g., otic, submandibular; see Figure 13-3, B, top). The parasympathetic postganglionic neurons project to smooth muscle and glandular targets in the head (e.g., ciliary muscle, parotid gland). Preganglionic axons leaving through cranial nerve X travel all the way to the body cavity to synapse in more diffuse parasympathetic ganglia located close to, or within, thoracic and abdominal viscera (see Figure 13-3, B, bottom). The short postganglionic neurons control the smooth muscle, cardiac muscle, and glandular cells of these organs. Parasympathetic preganglionic axons leaving through sacral spinal nerves depart to form pelvic nerves that synapse in diffuse parasympathetic ganglia residing close to, or within, pelvic viscera (e.g. rectum, bladder; see Figure 13-3, C). The short postganglionic neurons control these organs, as well as erectile tissue of the genitals. Most viscera receive both sympathetic and parasympathetic innervation (see Figure 13-2). Although the parasympathetic system originates in brainstem and sacral regions, it can provide parasympathetic innervation to organs in the thoracic and lumbar parts of the body, as just noted, by way of the vagus nerve (cranial nerve X). The sympathetic thoracolumbar system can influence organs in cranial and sacral regions by way of preganglionic sympathetic axons that travel to sympathetic postganglionic neurons in cervical and sacral regions of the sympathetic ganglion chain (see Figure 13-3, A, asterisk). Although blood vessels in all parts of the body receive sympathetic innervation, which most commonly produces vasoconstriction, most do not receive parasympathetic innervation (except those in glands and the external genitals). As described in Chapter 5, acetylcholine is the neurotransmitter at the somatic neuromuscular synapse. Acetylcholine is also released by the preganglionic neurons at all autonomic ganglia (Figure 13-4). Parasympathetic postganglionic neurons release acetylcholine as well, onto their target organs. Acetylcholine-releasing synapses are often called cholinergic. Most anatomically sympathetic postganglionic neurons secrete norepinephrine onto their targets. Norepinephrine-releasing synapses are often called adrenergic. However, in several species, anatomically sympathetic postganglionic neurons traveling to sweat glands secrete acetylcholine, as do some of the sympathetic postganglionic neurons to blood vessels in skeletal muscle, where they can produce vasodilation. Acetylcholine stimulates two different types of receptors (see Figure 13-4). Muscarinic acetylcholine receptors are G-protein–coupled receptors (GPCRs; see Chapter 1) found on all the target cells stimulated by postganglionic parasympathetic neurons and by cholinergic postganglionic neurons of the sympathetic nervous system. Faster acting nicotinic receptors are ligand-gated ion channels (see Chapter 1) found at all synapses between autonomic preganglionic and postganglionic neurons and at the somatic neuromuscular junction. Acetylcholine and norepinephrine can also be found in the enteric nervous system: acetylcholine is released by excitatory enteric neurons of the gut (see Chapter 27), and postganglionic sympathetic neurons can release norepinephrine into enteric neuronal plexuses to induce inhibition. Like the sympathetic/parasympathetic systems, various enteric neurons also employ vasoactive intestinal peptide, neuropeptide Y, ATP, and nitric oxide. However, the variety of neurotransmitters other than acetylcholine and norepinephrine, employed by neurons of the enteric nervous system, is much more extensive than that found among the sympathetic and parasympathetic systems.
The Autonomic Nervous System
The Peripheral Autonomic Nervous System Differs from the Somatic Motor System in a Number of Important Ways
The Peripheral Autonomic Nervous System Has Two Subdivisions That Originate in the Central Nervous System and One That Does Not
The Sympathetic Nervous System Originates from the Thoracolumbar Spinal Cord
The Parasympathetic Nervous System Arises from the Brainstem and Sacral Spinal Cord
Most Sympathetic and Parasympathetic Neurons Secrete Either Acetylcholine or Norepinephrine as a Neurotransmitter
Acetylcholine and Norepinephrine Have Different Postsynaptic Receptors
Neurotransmitters Other Than Acetylcholine and Norepinephrine Play Some Role in Peripheral Autonomic Function
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree