Fig. 1.
An anorectic anx/anx mouse and a +/+ mouse, age 17 days.
2.2 Genetics
The anx mutation arose spontaneously in the second filial generation (F2) of a cross between DW/J and an inbred strain, which was derived from a cross between an inbred Swiss stock and Mus musculus poschiavinus. The male anx carrier was crossed with a B6C3H-a/a F1 female, and the mutation has since been maintained on this background (45). The anx interval was initially mapped to a region approximately 20 cM proximal to the agouti locus, on chromosome 2 (Chr. 2) (45). By positional cloning, the locus was later narrowed down to a 0.2 cM interval between markers D2Mit133 and Jojo5, 1 and the anx mutation was found to cosegregate with markers D2Mit104, D2Mit395, and Jojo8 (Fig. 2) (46). The interval includes approximately 40 identified genes.
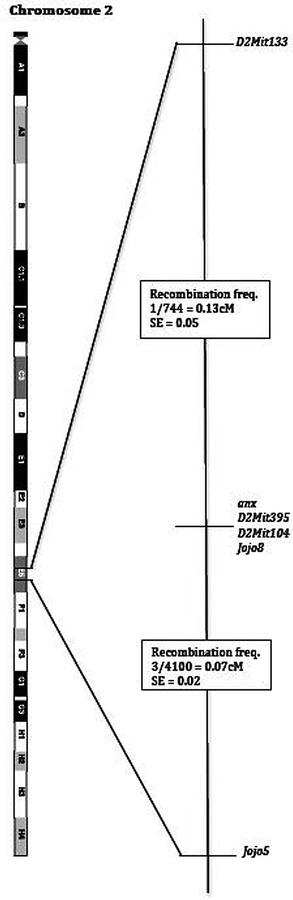

Fig. 2.
Mapping of the anx mutation.
2.3 Expression and Sequencing
Using an Affymetrix microarray analysis of the Arc, we identified a gene corresponding to one of the assembly factors for Complex I in the oxidative phosphorylation system (OXPHOS), Ndufaf1, to be downregulated in the anx/anx mouse (46). The downregulation was confirmed with both Western blot in the brain, and with real-time PCR in the brain, pancreas, liver, and lung. Since Ndufaf1 is mapped to the anx gene interval, we considered it a strong anx gene candidate. However, when sequencing the gene, both genomic (exons) and cDNA, no unique alterations in the anx/anx mice have been identified. The mutation does, consequently, appear to be located in a regulatory element of the Ndufaf1 gene.
3 Notes
3.1 Neurochemical Aberrances
Histochemical studies of the anorectic anx/anx mouse have revealed several aberrances in transmitter and neuropeptidergic systems, particularly in systems important for the regulation of food intake and energy metabolism, i.e., energy homeostasis (summarized in Table 1) (14, 42, 47–54).
Table 1
Neurohistochemical aberrances in hypothalamus of the anx/anx mice, at P21
Marker | Immunohistochemistry | In situ hybridization | |
|---|---|---|---|
NPY | ↑ Cell number/intensity | ↓ Fiber density | ↑/No change mRNA |
AGRP | ↑ Cell number/intensity | ↓ Fiber density | ↑ mRNA |
ACTH | ↓ Cell number | ||
α-MSH | ↓ Fiber density | ||
CART | |||
POMC | ↓ mRNA | ||
Serotonin | ↑ Innervation of Arc | ||
Y1 | ↓ Cell number | ↓ Dendrite density | ↓ mRNA |
Y2 | ↓ mRNA | ||
Y5 | ↓ mRNA | ||
The Arc is central in the energy homeostatic processes (1–12, 55–59). It harbors, in particular, two neuronal populations important for the regulation of food intake and energy metabolism (Fig. 3). One population coexpresses the orexigenic neuropeptides neuropeptide Y (NPY) and agouti-gene-related transcript (AGRP). Whereas AGRP is selectively synthesized in these Arc neurons, NPY is expressed in multiple, widely distributed cell groups (50, 65–67). Thus, AGRP is an ideal marker for studies on the distribution of AGRP/NPY projections in the brain. The other population coexpresses the anorexigenic cocaine- and amphetamine-regulated transcript (CART) and proopiomelanocortin (POMC), a precursor protein generating alpha-melanocyte stimulating hormone (α-MSH). The AGRP/NPY neurons are known to partly be gamma-aminobutyric acid (GABA)-ergic (68), the POMC/CART cells glutamatergic (69). Both the AGRP/NPY and POMC/CART neurons are affected by peripheral hormones, e.g., leptin and insulin, which are able to enter the Arc via a partly permeable blood–brain barrier in the Arc median eminence complex. The signal is propagated further via extensive projections from these Arc neurons to other hypothalamic areas, as well as areas outside the hypothalamus (50). Leptin and insulin bind to their respective receptors on the AGRP/NPY and POMC/CART neurons, affecting their activity by inhibiting the former and activating the latter (3, 5, 70, 71). It has also been established that the GABA/NPY/AGRP neurons innervate the POMC/CART neurons (72). Due to their roles in the regulation of food intake, both the AGRP/NPY and POMC/CART neurons have been extensively studied in anorectic anx/anx mice (42, 44, 47, 49, 51, 54).
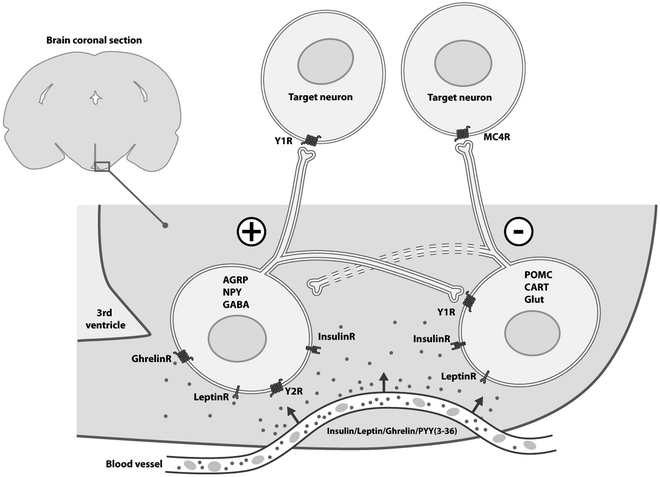

Fig. 3.
The arcuate nucleus (Arc) is located in the most ventral, medial aspects of the hypothalamus, adjacent to the third ventricle and the median eminence, and ventral to the ventromedial nucleus. It harbors a large number of neuron populations characterized by different neurochemical markers, in many cases coexisting (60, 61). There are two prominent cell groups that have received much attention due to their involvement in control of food intake: the Neuropeptide Y (NPY)/agouti-gene-related peptide (AGRP) neurons (ns), in part gamma-aminobutyric acid (GABA)-ergic (NAGns), and the pro-opiomelanocortin (POMC)/cocaine- and amphetamine-regulated transcript (CART) neurons, in part glutamatergic (Glut; PCGns). In contrast to many other neurons in the Arc, they do not project to the external layer of the median eminence, but their axons are directed into the brain, targeting both more closely located hypothalamic nuclei such as the paraventricular nucleus, and the perifornical area, harboring neurons expressing orexin/hypocretin and the melanin-concentrating hormone (MCH), but also sending axons to the lower brain stem such as the solitary tract nucleus, an input station for the vagus nerve and targets for blood-borne hormones like cholecystokinin, conveying both catabolic and anabolic information. The NAGns stimulate food intake (+), whereas the PCGns exert the opposite effect (−). The NAGns also innervate the PCGns, releasing NPY acting on inhibitory postsynaptic Y1 receptor (Y1R) on the PCGns. A reciprocal innervation, that is PCGns innervating the NAGns, is probably of less importance. Both NAGns and PCGns express leptin (cytokine receptor-type) and insulin (tyrosine kinase-type) receptors, whereas Y2 and ghrelin receptors are only found on NAGns. Since the Arc partly is outside the blood–brain barrier, leptin, insulin, ghrelin and PYY(3-36) can, even if large molecules, access the Arc neurons. However, active transport molecules may also be involved. This is a highly simplified drawing. For more detailed information and references, see (1–19, 62–64). MC4R melanocortin 4 receptor.
Immunohistochemistry analyses, using antibodies raised against NPY or AGRP, have revealed a reduced density of immunoreactive fibers in all projection areas studied, including the paraventricluar nucleus of hypothalamus (PVN), the lateral hypothalamic area (LHA), the dorsomedial hypothalamic nucleus (DMH), and the Arc, when comparing anx/anx mice and +/+ mice at P21 (50, 54). In addition, both peptides show a dramatic increase in number of cell bodies and in their staining intensity in Arc, resembling what in wild type is only seen after colchicine injection. Colchicine is a mitosis inhibitor and arrests centrifugal axonal transport, resulting in accumulation of molecules and organelles synthesized in the cell body (73). In situ hybridization studies of NPY and AGRP mRNA levels have been conflicting (51, 53, 54). Broberger et al. (54) concluded that there was no difference in mRNA levels of NPY in anx/anx when compared with +/+ mice at P21, which was confirmed by Jahng et al. (53). However, a later study showed an increased expression of both AGRP and NPY mRNA in anx/anx mice of the same age as in the previous studies (51). One explanation for the aberrances in the AGRP/NPY system is that loss of AGRP (and NPY) in the nerve terminals is due to increased release, which is then compensated for by increased synthesis/transcript levels. However, increased transcript levels can also be due to axonal degeneration, followed by feedback-induced increase in neuropeptide synthesis.
Immunohistochemistry with antibodies against α-MSH, one of the peptides from the precursor protein POMC, show markedly attenuated immunoreactive fibers in anx/anx hypothalamus. This is, as expected, accompanied by reduced numbers of immunoreactive cell bodies for ACTH, another fragment generated from the POMC precursor. Furthermore, immunohistochemistry for the NPY receptor (Y1), which decorates the soma and dendrites of POMC/CART neurons (74), confirms these results by showing a reduced density of both immunoreactive dendrites and cell bodies in anx/anx hypothalamus, suggesting atrophy or degeneration (Fig. 4) (47).
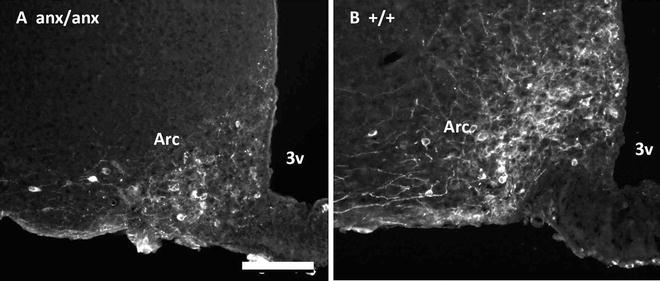

Fig. 4.
Immunohistochemistry for Neuropeptide Y receptor 1 (Y1), a marker for POMC/CART neurons in Arc of an anx/anx (a) or +/+ mouse (b). Note the reduced density of Y1-immunoreactive cell bodies and dendrites in the anx/anx mouse. 3v third ventricle; Arc arcuate nucleus. Scalebar = 10 μm.
In addition, two studies have reported serotonergic hyperinnervation of Arc, as well as the olfactory bulb, frontal cortex, hippocampus, and cerebellum in anx/anx mice (52, 53). Serotonin decreases food intake, and accordingly increased serotonergic innervation of Arc fits the anorectic phenotype of these mice. It is also possible that the reported alterations in the serotonergic system affect the abnormal motor behavior of the anx/anx mouse, e.g., head weaving, tremors, and uncoordinated gait. Maltais and colleagues report that 15-day-old normal mice that are treated with a serotonin precursor display these type of behaviors, and treating anx/anx mice with a serotonin antagonist has been shown to stabilize their motor abnormalities (45).
Abnormal dopaminergic neurotransmission has been demonstrated in the anx/anx mouse (75). In this study, a decrease of dopamine and its metabolites was detected in the striatum of anx/anx mice. The Na+, K+-ATPase activity, which is normally inhibited by dopamine, was upregulated in striatum of the anx/anx mouse, and isolated neostriatal neurons failed to respond to exogenous dopamine (75).
Finally, increased expression of neurotrophin tyrosine kinase receptor 3 (NTRK3) has been detected in the hypothalamus, but not in the cortex, of the anx/anx mouse. Interestingly, the NTRK3 gene has also been associated with eating disorders in humans (76).
3.2 Postnatal Development of Food-Intake-Regulating Systems in the anx/anx Mouse
Using AGRP as a marker for the Arc NPY neurons, we have studied the postnatal development of this orexigenic system in the anx/anx mouse. The AGRP/NPY system is known to develop postnatally in mice, i.e., there is a continuous increase in fiber density during the first 3 weeks of life, reaching adult appearance by P21 (22, 23, 77). In anx/anx mice, this system initially appears to develop as in wild-type mice, but around P12-15 the normal increase in fiber density ceases. Between P15 and P21 the amount of fluorescent fibers is reduced in the anx/anx mouse (exemplified by PVN in Fig. 5a, b, e, f) (42). Whether this represents increased release of AGRP and NPY or degeneration remains to be determined.
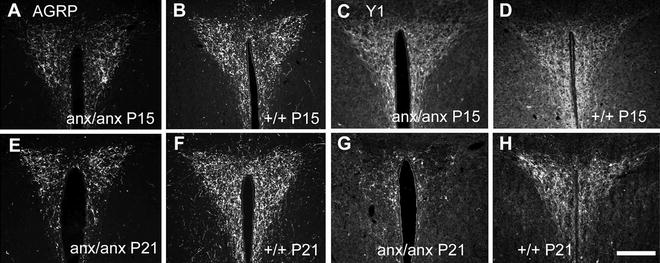

Fig. 5.
Immunohistochemistry for agouti gene-related protein (AGRP; a, b, e, and f) and Neuropeptide Y receptor 1 (Y1) (c, d, g, and h) in paraventricular nucleus (PVN) of anx/anx (a, c, e, and g) and +/+ mice (b, d, f, and h) at P15 (a–d) and P21 (e–h). Note that a reduced density of AGRP-immunoreactive fibers in the anx/anx mice is visible already at P15, while such a reduction in Y1-immunoreactive processes is present first by P21. Scalebar = 200 μm.
The development of the anorexigenic POMC system was also studied, using the NPY receptor Y1 as a marker. Here we concluded that the POMC system develops as in normal mice until after P15, thus a reduced density of Y1-positive fibers was detected first by P21 (exemplified by PVN in Fig. 5c, d, g, h) (44). In fact, the POMC neurons do not show signs of degeneration until P21, thus following the disappearance of AGRP/NPY fibers, which are already showing reductions at P12-15 (42).
From P8, anx/anx mice have significantly reduced serum leptin levels (49), which is not surprising, keeping in mind that this is an adipocyte-derived hormone. Based on leptin’s role in the postnatal development of the food-intake-regulating neurons in Arc (20, 21), one can speculate that the low levels of leptin contribute to the aberrances, e.g., neurochemical, neurodevelopmental, and neurodegenerative processes, seen in these circuits in the anx/anx mouse. Bouret et al. showed leptin’s neurotrophic effect on the Arc neurons through studies on the obese leptin-deficient ob/ob mouse (20). The ob/ob mouse shows an abnormal development of Arc projections, resembling the pattern seen in anx/anx mice. By injecting ob/ob mice postnatally with leptin, they were able to normalize the development of these projections.
3.3 Hypothalamic Neuroinflammation
Several studies have indicated a relation between hypothalamic inflammation and the anorexia of anx/anx mice. Laucher and colleagues, using gene expression analyses of the hypothalamus, concluded that an inflammatory response is involved in the phenotype (41). In a second gene expression study of the anx/anx hypothalamus, Mercader and colleagues also concluded that the phenotype is related to changes in several inflammatory genes (43). In parallel, we have shown a strong activation of microglia cells specific for regions to which the food-intake-regulating AGRP neurons project, in particular hypothalamic regions, in anx/anx mice. Signs of activated microglia in these regions have been documented from P12, and progressively increase, reaching strong activation at P21, exemplified by LHA in Fig. 6 (42).
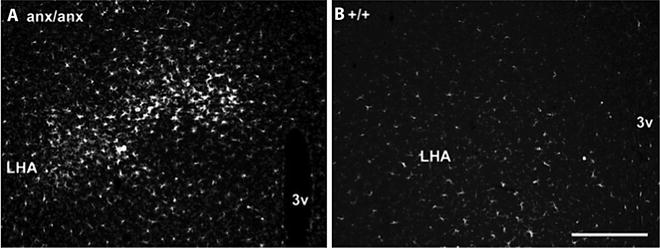

Fig. 6.
Activated microglia cells, visualized by ionized calcium-binding adapter 1 (Iba1) immunohistochemistry, in hypothalamus of an anx/anx (a) or +/+ mouse (b). LHA lateral hypothalamic area; 3v third ventricle. Scalebar = 200 μm.
Microglia cells are normally activated in the central nervous system in response to neuroinflammation and/or neurodegeneration (78–80), and these results indicate that such processes occur in the hypothalamus of the anx/anx mouse. In addition, we have provided evidence of expression of major histocompatibility (MHC) complex I by glia cells located in the areas to which the AGRP neurons project (LHA, PVN, and DMH) as well as by Arc neurons (44). MHC class I is well known to be expressed by activated microglia in response to inflammatory stimuli, and by neurons during pathological conditions, such as acute inflammation (81–85).
Inflammatory mechanisms in the hypothalamus have been related to the impaired signaling seen after feeding with high-fat diet (86), obesity (87), and in cachexia (88). Thus, it seems that conditions of both excess and shortage of energy are coupled with hypothalamic mechanisms involving inflammation (89). This is paradoxical, and an extensive evaluation of the inflammatory progression in the different conditions is needed to understand this complex relationship between energy balance and hypothalamic inflammation. It is possible that studies of the anx/anx mouse may help resolve this paradox.
3.4 Hypothalamic Neurodegeneration
In addition to the signs of neuroinflammation and the loss of AGRP and NPY in nerve terminals, several observations have indicated neurodegenerative processes in the hypothalamus of the anx/anx mouse. Initially, Broberger and coworkers observed a reduced staining of POMC cells with an apparent retraction/shrinkage of Y1-immunoreactive dendrites, suggesting a degeneration of the POMC/CART system of anx/anx mice at P21 (47). It should be noted that this view was based on data with a chemical marker, the Y1 receptor protein. Thus, no “structural” degenerative changes were demonstrated, and a downregulation of Y1 receptor synthesis could not be excluded. Interestingly, the loss of AGRP/NPY in terminals precedes the signs of degeneration seen in the POMC system. It is thus possible that the POMC system degenerates as a consequence of the lost input from the AGRP/NPY neurons.
The finding of a strong activation of microglia cells, overlapping both in time and region with the reduced density of fibers immunoreactive for AGRP (42), gave further support to the neurodegeneration hypothesis in the anx/anx Arc. These results are in agreement with results from Wu et al., showing that ablation of AGRP neurons results in gliosis in the target areas (90). Moreover, the expression of MHC I by Arc neurons (44) may also support such a process being involved, since MHC class I has been shown to be expressed by degenerating (91, 92) or lesioned (93) neurons. We have detected an increased number of terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL)-positive neurons in anx/anx hypothalami as well as possible signs of what Ribak et al. call “microglia-associated cell death” (44, 94). This process of cell death appears different from both apoptosis and necrosis, whereby a microglia cell appears to embrace a neuron and create “holes” in the plasma membrane, resulting in a watery cytoplasm and nucleoplasm, and damaged organelles. We have also detected NPY-positive fibers expressing activated caspase 6 (44), a marker for axonal degeneration (95). Taken together, all of these results strongly indicate degenerative processes in the hypothalamus of the anx/anx mouse.
With regard to neurodegeneration, it should be mentioned that bromodeoxyuridine- and TUNEL-labeling studies have shown increased cell proliferation and apoptosis in dentate gyrus of anx/anx mouse (96). A differential display analysis of the whole brain identified, among other genes, the apoptotic protease activating factor 1 as being regulated by the anx mutation (97). Last, our microarray analysis of the Arc region, mentioned previously, indicated several genes related to apoptosis/cell death/degeneration deregulated in the anx/anx mouse (Table 2).
Table 2
Genes related to degeneration/cell death/apoptosis with altered expression in the anx/anx Arc
Symbol | Full gene name | Fold change |
|---|---|---|
HLA-C | Major histocompatibility complex, class I, C | 7.5 |
USP18 | Ubiquitin specific peptidase 18 | 5.6 |
STAT1 | Signal transducer and activator of transcription 1, 91kDa | 5.6 |
IFIH1 | Interferon induced with helicase C domain 1 | 4.6 |
B2M | Beta-2-microglobulin | 3.3 |
GFAP | Glial fibrillary acidic protein | 2.8 |
CTSS | Cathepsin S | 2.3 |
YWHAZ | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta polypeptide | 2.2 |
PIM3 | pim-3 Oncogene | 2.1 |
BCL2L11 | BCL2-like 11 (apoptosis facilitator) | 2.1 |
HMGB1L1 | High mobility group box 1 pseudogene 1 | 2.0 |
GSTM5 | Glutathione S-transferase mu 5 | 1.8 |
CNP | 2′,3′-Cyclic nucleotide 3′ phosphodiesterase | 1.7 |
SOD1 | Superoxide dismutase 1, soluble | 1.7 |
VAMP3 | Vesicle-associated membrane protein 3 (cellubrevin) | 1.7 |
HINT1 | Histidine triad nucleotide-binding protein 1 | 1.6 |
EGR1 | Early growth response 1 | 1.6 |
MBP | Myelin basic protein | 1.6 |
ZNF622 | Zinc finger protein 622 | 1.6 |
NDN | Necdin homolog (mouse) | 1.6 |
GLO1 | Glyoxalase I | 1.5 |
MT2A | Metallothionein 2A | 1.5 |
CSF1R | Colony stimulating factor 1 receptor | 1.5 |
FKBP1A | FK506-binding protein 1A, 12kDa | 1.5 |
DTYMK | Deoxythymidylate kinase (thymidylate kinase) | 1.5 |
LAMP1 | Lysosomal-associated membrane protein 1 | 1.5 |
RPS6 | Ribosomal protein S6 | 1.5 |
HK1 | Hexokinase 1 | 1.5 |
CTTN | Cortactin | 1.5 |
GJA1 | Gap junction protein, alpha 1, 43kDa | 1.5 |
PAK3 | P21 protein (Cdc42/Rac)-activated kinase 3 | −1.5 |
PNP | Purine nucleoside phosphorylase | −1.5 |
ABCD2 | ATP-binding cassette, subfamily D (ALD), member 2 | −1.5 |
GABRB2 | Gamma-aminobutyric acid (GABA) A receptor, beta 2 | −1.5 |
CXCL12 | Chemokine (C–X–C motif) ligand 12 | −1.5 |
LGALS1 | Lectin, galactoside-binding, soluble, 1 | −1.5 |
MAP2K4 | Mitogen-activated protein kinase kinase 4 | −1.6 |
GRM3 | Glutamate receptor, metabotropic 3 | −1.6 |
TNFAIP8 | Tumor necrosis factor, alpha-induced protein 8 | −1.7 |
PPARGC1A | Peroxisome proliferator-activated receptor gamma, coactivator 1 alpha | −1.7 |
TUBB3 | Tubulin, beta 3 | −1.9 |
PLA2G7 | Phospholipase A2, group VII (platelet-activating factor acetylhydrolase, plasma) | −1.9 |
SLC1A2 | Solute carrier family 1 (glial high affinity glutamate transporter), member 2 | −2.1 |
NDUFAF1 | NADH dehydrogenase (ubiquinone) 1 alpha subcomplex, assembly factor 1 | −2.2 |
FSTL1 | Follistatin-like 1 | −2.3 |
IGFBP4 < div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|