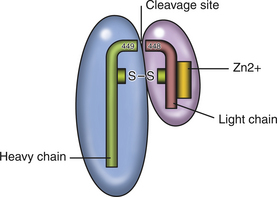
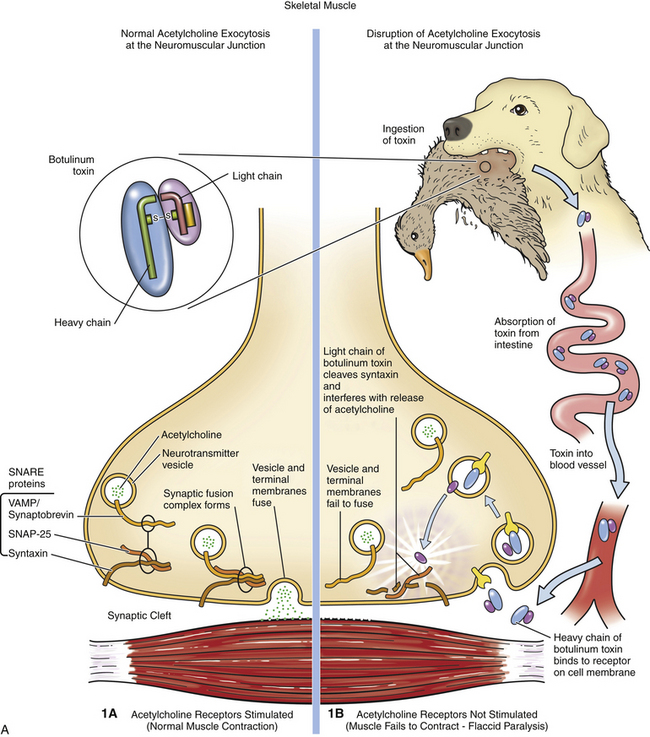
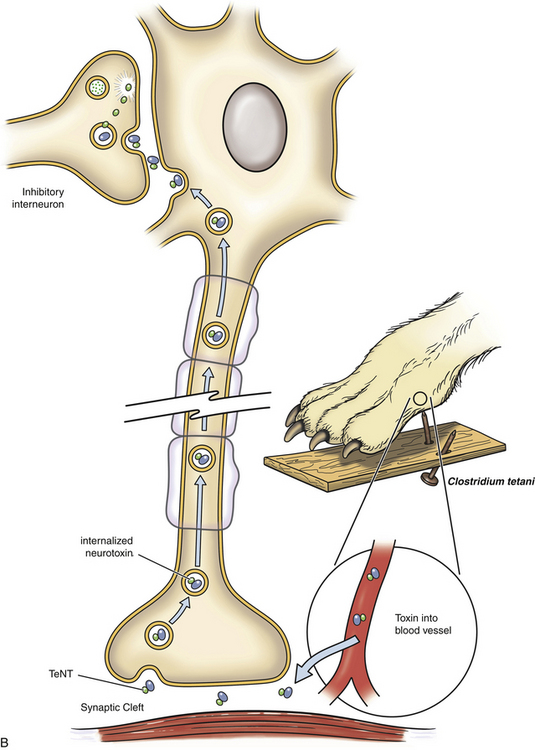
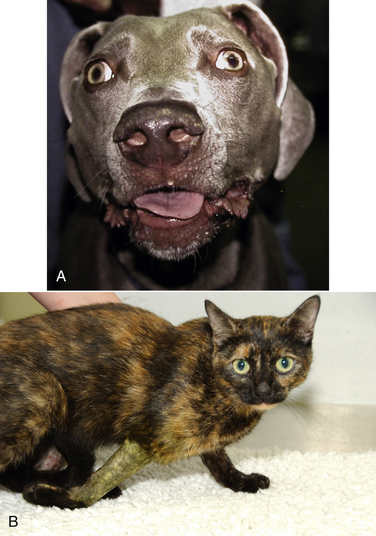
Chapter 54 In dogs and especially cats, botulism is rare and usually follows ingestion of preformed neurotoxin within carrion, especially waterfowl or poultry carrion. Disease in dogs can coincide with outbreaks of botulism in waterfowl. Botulism outbreaks in dogs have been reported in hunting foxhounds.3,4 Most affected dogs are medium- to large-breed, intact male or intact female dogs that vary in age. The only report of botulism in cats was an outbreak of disease that occurred after several cats were fed pelican carrion.5 The relative resistance of dogs and cats to botulism has been hypothesized to reflect an adaptive response to a carnivorous lifestyle. C. botulinum produces seven related toxins: A, B, C, D, E, F, and G. Most human botulism is caused by types A, B, and E.6 Botulism in dogs and cats almost always results from ingestion of toxin C,3,5,7–9 with the exception of a few dogs in the Senegal that had type D intoxication.10 C. tetani is widespread in the soil and feces of animals. It produces two toxins, tetanospasmin and tetanolysin. Tetanolysin does not appear to have clinical significance. Humans and horses are most susceptible to tetanus, and so widespread vaccination against the disease is performed in these species. Disease occurs occasionally in dogs and is rare in cats. The development of tetanus in dogs and cats usually follows introduction of spores into a penetrating wound, but more than one third of affected dogs have no known wound history (cryptogenic tetanus).11 Traumatic wounds of the feet, claws, or head; draining tracts associated with grass awn migration; animal bite wounds; tick bite wounds; bleeding claw trim wounds; wounds that result from chronic protozoal or fungal infections of the skin; teething wounds in dogs; and surgical wounds (which include spay and neuter operations) have all been implicated.11–14 Although dogs of any age, breed, or sex develop tetanus, disease occurs most often in young, large-breed, active, intact male dogs.11,13,14 Disease is seen in puppies as young as 8 weeks of age; some studies describe a significant proportion of affected dogs as less than 1 year of age. Disease is also more severe in young dogs.11 Cats are often young cats with outdoor access, but cats of any age have been affected.15 The toxins of C. tetani and C. botulinum have similar structures and mechanisms of action, even though they cause diseases that contrast sharply with one another. Both toxins are among the most potent toxins known and act at femtomolar concentrations. They consist of two polypeptide chains, a heavy (H) chain and a light (L) chain, joined by a disulfide bond (Figure 54-1). The heavy chain attaches to presynaptic nerve terminal membrane receptors, although the precise receptors have not been identified because of the extremely low concentrations at which these toxins act. Variation in receptor binding affinities may explain differences in species susceptibility to clostridial neurotoxins. The toxin is then internalized via receptor-mediated endocytosis into the presynaptic nerve terminal, where it has a local impact on nerve cell function (botulinum toxin) and also travels by retrograde axonal transport to the nerve cell body and diffuses into inhibitory interneurons, where it impacts inhibitory interneuron function (tetanospasmin). The L chain, a zinc-dependent matrix metalloproteinase, is released from the vesicle and subsequently cleaves one or more docking proteins. These proteins are critical for the release of neurotransmitters from synaptic vesicles into the synaptic cleft and are located on the membrane of synaptic vesicles (synaptobrevin) or the presynaptic cell membrane (syntaxin and SNAP-25). Synaptobrevin binds to the docking proteins located on the presynaptic membrane. The resulting complex (the SNARE complex) docks the synaptic vesicle at the proper location for fusion and exocytosis of neurotransmitter molecules into the synaptic space (Figure 54-2). Tetanospasmin cleaves synaptobrevin, whereas botulinum neurotoxin C cleaves syntaxin; other botulinum neurotoxins cleave synaptobrevin or SNAP-25. The process of recovery involves the sprouting of new presynaptic terminals, followed by recovery of the original synapse, after which new terminal outgrowths retract.16 C. botulinum spores germinate in anaerobic conditions and produce toxin. They grow best in temperatures that range from 30°C to 37°C. The toxin is ingested, survives the acid stomach conditions, and is absorbed into the bloodstream from the small intestine. It then travels to peripheral cholinergic synapses, which include the neuromuscular junction and autonomic synapses, and inhibits release of acetylcholine (ACh). This leads to ascending symmetrical flaccid paralysis, development of megaesophagus, and signs of autonomic dysfunction. Clinical signs usually occur within 12 to 72 hours after ingestion of botulinum toxin and may be complicated by signs of dietary indiscretion, although gastrointestinal signs may also reflect the presence of autonomic dysfunction. Disease progresses rapidly over 1 or 2 days, and then begins to resolve with supportive care. Death can result from respiratory muscle paralysis or aspiration of intestinal contents. Abnormalities in affected cats were ascending flaccid paralysis, lethargy, hypothermia, tachypnea, dehydration, and urinary incontinence.5 C. tetani spores are introduced into tissues where they germinate, replicate locally, and produce toxin in anaerobic conditions. Ingestion is not a route of transmission, because tetanospasmin is destroyed by gastrointestinal secretions. The toxin is produced as a single polyprotein that is cleaved extracellularly by a bacterial protease into the L and H chains, which remain connected by the disulfide bond. It then binds to presynaptic terminals of lower motor neurons and is internalized, travels up axons by retrograde axonal flow, and enters inhibitory interneurons in the brain and spinal cord, where it interferes with release of γ-aminobutyric acid (GABA) and glycine inhibitory neurotransmitters (Figure 54-2, B). Toxin can also spread hematogenously to the central nervous system (CNS) from the wound site. The result is unhindered excitation of motor neurons, spastic paralysis, and dysfunction of the sympathetic and parasympathomimetic nervous systems. Clinical signs occur 3 days to 3 weeks after a wound in both cats or dogs. Shorter incubation periods occur when the wound is proximal to the CNS and when a large amount of toxin is present. Paralysis may initially be localized to the wound site but can then generalize. Involvement of the head is common in dogs, and often owners of dogs with tetanus first notice an unusual appearance to their dog’s eyes, a “swollen” face, or evidence of dysphagia.11 Regurgitation or vomiting of food occurs in less than 20% of affected dogs, and a voice change or dysuria may be noted. Death occurs in severely affected dogs due to paralysis of respiratory muscles; infectious complications such as pneumonia; upper airway obstruction; the development of severe hyperthermia with disseminated intravascular coagulation (DIC), multiple organ failure, and/or pulmonary thromboembolism; and cardiac arrhythmias.11,13,14 Hiatal hernia and coxofemoral joint luxations have also been described as complications of tetanus in dogs.11,14,17 Disease in cats is usually localized to the limbs, but generalized tetanus can occur.15,18–21 Many affected cats have an initial history of disappearance for days to a week or more, after which lameness is noted. Physical examination findings in dogs with botulism depend on the severity of intoxication. Fever is absent unless aspiration pneumonia has developed. Mildly affected dogs show only ataxia or pelvic limb paresis, whereas dogs with severe intoxication are quadriplegic with absent voluntary motor activity and are unable to rise from lateral recumbency. The ability to wag the tail is often retained.3,8,9 Neurologic examination can reveal decreased cranial nerve function, especially facial nerve function and a decreased gag reflex, sometimes accompanied by ptyalism.3,7,9,22 A weak blink reflex can result in corneal ulceration. A mucopurulent ocular discharge may be present. Mydriasis with sluggish pupillary light reflexes has been described.3,22 Segmental reflexes may be intact, decreased, or absent, but pain sensation is preserved. Occasionally tachycardia and tachypnea are present. Tachypnea may result from diaphragmatic muscle weakness or secondary aspiration pneumonia. Increased lung sounds may also be present on thoracic auscultation of dogs with secondary aspiration pneumonia. Physical examination findings in one affected cat consisted of hypothermia, lethargy, dehydration, quadriplegia, and decreased segmental reflexes.5 In dogs with tetanus, involvement of the head and generalized disease occurs more often than occurs in cats. A wound may or may not be found. Dogs with localized forelimb tetanus have caudal retraction of the limb with extension of both the elbow and carpus.15 In dogs with generalized tetanus, a wrinkled forehead, erect ears, retracted lips (risus sardonicus), and prolapsed third eyelids are almost always present, usually in association with generalized muscle stiffness (see Figure 54-3, A). Miosis may be present. It may be difficult or impossible to open the mouth. Dogs that are ambulatory walk slowly with a stiff, stilted gait and can have a wide-based or “sawhorse” stance; voluntary movement of the head may occur slowly or appear difficult, and the tail may be erect. Laryngeal spasm and hypersalivation occur in approximately half of affected dogs.13,14 Reflexes are exaggerated or difficult to elicit. Severely affected dogs are anxious and hypersensitive, develop opisthotonos, vocalize, tremble or even have a seizure when stimulated, and may be hyperthermic due to increased muscle activity (up to 111°F or 43.9°C).11 A semicomatose state may even result. Abdominal palpation may reveal an enlarged bladder that is difficult to express, or palpation may not be possible because of abdominal muscle spasm. Hypoventilation may be present, or tetanic spasms may trigger episodes of dyspnea. At least a third of dogs are bradycardic or have bradyarrhythmias (which include atrioventricular block or sinus arrest), with heart rates that range from 40 to 60 beats/min; another third of dogs are tachycardic (>150 beats/min) or have variable heart rates, which may reflect autonomic dysfunction or pain.11 FIGURE 54-3 A, Image of a Weimaraner with generalized tetanus. The dog walked with a stiff gait and was salivating profusely. Risus sardonicus is evident. B, Three-year-old female domestic shorthair cat with localized tetanus that was seen for a 3-day history of right thoracic limb lameness. Extension of the shoulder and elbow and partial flexion of the carpal joint is present. (From Langner KFA, Schenk HC, Leithaeuser C, et al. Localised tetanus in a cat. Vet Rec 2011;169:126.) Cats with localized tetanus often have extensor rigidity of a single limb, on which a wound may be identified. When the forelimb is affected, spasm of the triceps muscle results in caudal extension of the limb with carpal flexion (in contrast to dogs) and elbow extension (Figure 54-3, B).20,23,24 The head and neck may be deviated toward the affected limb.15 When disease generalizes, the contralateral limb and subsequently all four limbs may be affected.20 Affected cats may be reluctant to move or hyperesthetic. Lockjaw (trismus), protrusion of the third eyelid, retracted lips (risus sardonicus), wrinkling of the forehead, or opisthotonos can occur in cats with generalized tetanus. Segmental reflexes may be exaggerated, or it may not be possible to elicit reflexes because of severe muscle spasticity. Dyspnea, tachypnea, and open-mouth breathing may occur.12 Cranial nerve responses are usually normal. Miosis may be present. Diagnosis of tetanus and botulism in dogs and cats is most often based on a consistent history and clinical abnormalities, exclusion of the few other possible causes of disease (e.g., with assays for myasthenia gravis in dogs with botulism), and sometimes electrodiagnostic testing. The primary differential diagnoses for botulism are acute polyradiculoneuritis and myasthenia gravis; paralytic rabies should also be considered. There are few other disorders other than strychnine poisoning that truly mimic tetanus. Serologic assays have been used to detect an antibody response to botulinum toxins. Finally, specific assays that detect botulinum toxin in serum, gastrointestinal contents, or food can be performed (Table 54-1). Complete Blood Count, Serum Biochemical Tests, and Urinalysis Routine laboratory test results in animals with tetanus or botulism are often normal, but stress hyperglycemia, mild to severe increases in the activity of serum creatine kinase (usually <20,000 U/L but sometimes >200,000 U/L, reference range 46-320 U/L), mildly increased AST activity, and myoglobinuria may be present in animals with tetanus.11,14 Dehydration may result in hemoconcentration or prerenal azotemia. Animals with wounds or aspiration pneumonia may have an inflammatory leukogram. Severe hyperthermia in dogs with tetanus may lead to laboratory evidence of multiple organ dysfunction, with moderate to severe hypoalbuminemia, azotemia, and/or increased liver enzyme activities and hyperbilirubinemia. Thrombocytopenia and coagulation test abnormalities consistent with DIC may also be present in these dogs.
Tetanus and Botulism
Etiology and Epidemiology
Botulism
Tetanus
Clinical Features
Botulism
Tetanus
Physical and Neurologic Examination Findings
Botulism
Tetanus
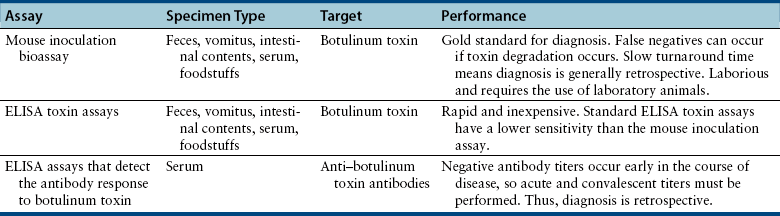
Diagnosis
Laboratory Abnormalities
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Tetanus and Botulism
Only gold members can continue reading. Log In or Register to continue