CHAPTER 19 Teratology
HISTORY OF TERATOLOGY
Teratology is the study of abnormal development. The idea that extrinsic influences ranging from ‘evil spirits’ to diet may influence the development of human and animal embryos has been held since Antiquity, but it became more precise in the nineteenth century with Etienne Geoffroy de Saint Hilaire, who studied in great detail several prenatal anomalies like cyclocephalia, anencephaly, and twin monsters and also coined the very term ‘teratology’. In the last century two sets of findings strongly influenced the establishment of teratology as a scientific discipline strongly connected to embryology. The first set concerned the deleterious effects of hyper- and hypovitaminosis A on mouse and pig embryos. The second, and more dramatic, set related to the effects of thalidomide, a sedative used to prevent morning sickness during early pregnancy in humans, and which resulted in devastatingly severe limb defects in numerous newborns in Europe during the late 1950s and early 1960s. Since then, the number of known and suspected teratogens has grown tremendously. Recently, the possibility that cloning by somatic cell nuclear transfer can cause severe malformations has caused concern, with developmental defects being attributed to incomplete reprogramming of the somatic cell genome by the oocyte cytoplasm (see Chapter 21).
CRITICAL PERIODS OF SUSCEPTIBILITY TO ABNORMAL DEVELOPMENT
CAUSES OF MALFORMATIONS
Significance of genotype
Genetically based malformations result from abnormalities of chromosomal division or mutations of genes. Abnormal genes may either be the direct cause of an anomaly, or may induce the anomaly indirectly by affecting the susceptibility of the embryo to an environmental insult. Thus, the predisposition to an insult may be inherited.
Abnormal chromosome numbers
Quantitative changes in chromosomal numbers result in polyploidy or aneuploidy. In polyploidy, the chromosomal number is more than twice the haploid number of chromosomes of a given species. Most polyploid embryos are aborted early in pregnancy. Potential causes of polyploidy are fertilization of an egg by more than one sperm (polyspermy) or lack of separation of a polar body during meiosis of the oocyte. Embryos may also display mixoploidy, where a group of normal diploid cells is mixed with another portion of polyploid cells. This situation probably results from the lack of cytokinesis during initial mitoses. Interestingly, up to 25% of normal blastocysts recovered from cows are mixoploid and have a low percentage of polyploid cells, most of which become sequestered in the trophectoderm later in development. The incidence of mixoploidy is increased in embryos produced in vitro or by somatic cell nuclear transfer (see Chapter 21).
Numerical errors in the number of a particular chromosome result in aneuploidy. This is defined as a total number of chromosomes other than the usual haploid set for a given species. Monosomy is the lack of one member of a chromosome pair. In trisomy, a triplet instead of a normal chromosome pair occurs. Both events are typically the result of nondisjunction during meiosis. A number of conditions resulting from monosomy and trisomy are listed in Table 19-1. In most cases, embryos with monosomy of the autosomes and sex chromosomes cannot survive. However, in man as well as in domestic animals, individuals with monosomy of the X-chromosome (XO genotype) are viable. In humans, this condition is called Turner’s syndrome, characterized by a female phenotype with sterile ovaries.
Abnormalities caused by assisted reproductive technologies
Abnormally large offspring, with a body weight increased by 10–50%, are more frequently born following assisted reproductive technologies (see Chapter 21). In cattle and sheep this large offspring syndrome (LOS) is often associated with neonatal respiratory distress and perinatal death. The aetiology of LOS is not fully elucidated but it has been proposed that some growth factors in the sera used for embryo culture, co-culture of the embryos with feeder cells, an asynchronous uterine environment, or other unknown factors in assisted reproductive technologies may be involved.
Environmental factors
Chemical factors
Any chemical substance that has the potential to alter cellular function or which is cytotoxic has the potential of being teratogenic. The mechanisms that lead to teratogenesis vary. For example, some drugs can inhibit specific enzyme systems (e.g. carbonic anhydrase), interfere with DNA metabolism, or disturb particular metabolic activities. Retinoic acid, for instance, acts as a potent teratogen when taken orally. This compound produces a wide spectrum of defects, most of which are related to derivatives of the cranial neural crest. These involve facial structures, the heart, and the thymus. As described in Chapter 8, neural crest cells from rhombomeres are instrumental in patterning many structures of the face and neck and contribute to the outflow tract of the heart. Retinoic acid affects the expression of Hox genes in the cranial and pharyngeal regions causing alterations of the anterior rhombomeres and of the neural crest cells derived from them.
It has been shown that at defined exposure levels and at critical stages of development, several therapeutic drugs can also be potentially teratogenic (Table 19-2). Special caution should be taken when prescribing cytotoxic drugs such as antimitotic or anthelminthic agents to pregnant animals. For some of these drugs, the teratogenic effects on the developing embryo are well understood. However, for many substances it is still not clear in which species and at what concentrations they might be teratogenic. For instance, the classic teratogen thalidomide is highly teratogenic in humans, rabbits, and some primates, but not in the commonly used laboratory rodents. The use of some antibiotics during pregnancy has been associated with congenital defects: streptomycin in high dose can result in inner ear deafness; tetracycline can cross the placental barrier and cause a yellowish discolouration of teeth (and, in high doses, interfere with enamel formation) when given late in pregnancy.
Many poisonous plants containing toxic or teratogenic compounds have been implicated in congenital defects in herbivorous animals. Some of these compounds are listed in Table 19-3. The malformations produced vary widely and include skeletal deformities, limb defects, cyclopia and cleft palate. As with other agents, there are distinct times during development when the embryo or fetus is particularly susceptible to certain plant teratogens. A well-known example is the effect of Veratrum californicum which induces congenital cyclopia if consumed by ewes at around Day 14 of pregnancy.
Infectious agents
Most infectious diseases that cause birth defects are viral. The pathogenicity of a virus, the stage of gestation at which infection occurs, and the immunological competence of the fetus determines the outcome of in utero viral infections. A summary of infectious virus diseases that can cause birth defects in domestic animals is given in Table 19-4. During early development, the zona pellucida is protective against viral infections, but when the blastocyst hatches from the zona pellucida, the embryo becomes vulnerable to attack by viruses. Many viral infections are toxic and/or lethal to the embryo. Later, the placental barrier prevents viral infections to some degree but many viruses can cross the placental barrier. Therefore maternal viral infections at critical stages of development can be a serious cause of malformations. Infection of pregnant sheep, cattle, and goats with Akabane virus causes teratogenic defects (e.g. arthrogryposis; hydranencephaly) that are closely related to fetal age at time of infection.
Table 19-4: Common teratogenic viruses
| Virus | Species affected | Effects |
|---|---|---|
| Akabane virus | Cattle, sheep, goat | Brain defects (hydranencephaly, porencephaly), limb defects (arthrogryposis) |
| Bluetongue virus | Sheep, cattle, goats | Brain, spinal cord, limb defects |
| Border disease virus | Sheep, goats | Wide range of embryonic and fetal changes, skeletal growth retardation, cerebellar dysplasia |
| Bovine rhinotracheitis | Cattle | Embryotoxic |
| Bovine viral diarrhoea | Cattle | Embryonic death; abnormalities of the central nervous system and ocular abnormalities |
| Classical swine fever | Pigs | Malformation of the central nervous system (cerebellar and spinal hypoplasia) |
| Equine rhinopneumonitis | Horses | Embryotoxic |
| Equine encephalitis | Horses | Limb defect |
| Feline panleukopaenia | Cats | Cerebellar defects, retinal dysplasia |
| Herpesvirus 2 | Dogs | Eye, brain defects |
| Japanese encephalitis | Pigs, sometimes horses | Hydrocephalus, cerebellar hypoplasia, hypomyelinogenesis |
| Porcine herpesvirus 1 | Pigs | Abortion, stillborn or mummified piglets |
| Porcine parvovirus | Pigs | Abortion, stillborn or mummified piglets |
| Rift valley fever virus | Sheep, cattle, goat | Arthrogryposis, hydranencephaly, cerebellar hypoplasia, microcephaly |
| Rubella | Monkey, rabbit | Heart, eye, brain and skeletal defects |
CLASSIFICATION OF MALFORMATIONS
The major types of developmental disturbances found in domestic animals include:
Failure to fuse or close
Fusion is a basic morphogenic process involved in the formation of many structures (Fig. 19-1). Therefore, failure of completion of a fusion process is one of the more important types of developmental defects. Examples are cleft palate, defects in the diaphragm, and various septal defects in the heart. A classic case is the failure of tube formation in spina bifida anomalies, in which fusion of the neural tube is incomplete.
Disturbances in tissue resorption
Some structures present in the early embryo must be resorbed for normal development. Examples include the resorption of the oro-pharyngeal membrane and the cloacal membrane that cover the future oral and anal openings. Anal atresia is a common anomaly in piglets.
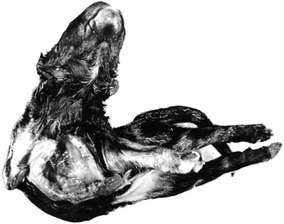
Duplication and reversal of asymmetry (Figs 19-2, 19-3, 19-4, 19-5)
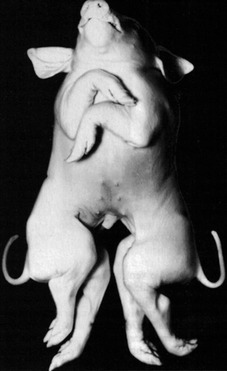
Fig. 19-2: These twin pigs are conjoined at the thorax and head, resulting in a cephalothoracopagus.
Courtesy Sinowatz and Rüsse (2007).
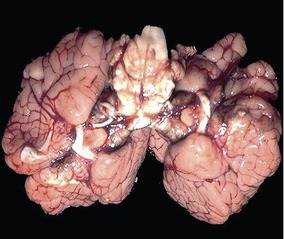
Fig. 19-4: The brain of the calf shown in Fig. 19-3 shows a doubling of the hemispheres but only one brain stem.
CONGENITAL MALFORMATIONS OF THE CARDIOVASCULAR SYSTEM
Dextrocardia
Dextrocardia is a malformation in which the heart lies more to the right side of the thorax than to the left. This condition is caused early in development, when the heart tube loops to the left instead of the right. Dextrocardia may be associated with situs inversus, a complete reversal of asymmetry in all organs. In this anomaly the chambers normally on the left and right are reversed in position and the ductus arteriosus, aortic arches and pulmonary veins form on the right. The condition can be compatible with normal life. In other cases sidedness is random and only some organs show a reversed position. This condition is called heterotaxy and is associated with an increased incidence of other malformations, especially heart defects.
Acyanotic heart malformations
Obstruction of the left ventricular outflow (aortic stenosis)
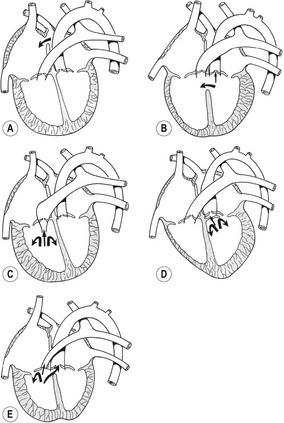
Three different grades of severity of LVOT obstruction have been described:
Pulmonary stenosis
Pulmonary stenosis consists of a narrowing of the pulmonary outflow and can occur at several possible sites. Although subvalvular and supravalvular forms of pulmonary stenosis have been reported, the most common form of the right ventricular obstruction in dogs is pulmonary valve dysplasia which can be present as a single defect or associated with other heart defects. A pulmonary artery atresia represents the extreme form of obstruction of the right ventricular tract. The nature of the obstruction can be diagnosed by echocardiography. When valvular fusion exists, the semilunar cusps are fused towards the tips of the leaflet, resulting in doming of the valves. When hypoplastic type of pulmonary stenosis is present, the valve appears thickened and immobile and the annulus may be narrow and hypoplastic. The main haemodynamic effects of pulmonary stenosis can be visualized as concentric right ventricular hypertrophy, paradoxical septal motion, and reduced size of the left atrium and left ventricle in moderate to severe pulmonary stenosis. Dilatation of the main pulmonary artery can be seen distally to the obstruction, close to the bifurcation. Spectral Doppler echocardiography of pulmonary stenosis will record increased peak pulmonary artery velocity (>1.6 m/s) and often prominent pulmonary insufficiency. A Doppler gradient across the stenosis of <50 mm Hg is considered as mild pulmonary stenosis. Gradients between 50 and 100 mm Hg are moderate in severity and higher gradients are associated with severe pulmonary stenosis.
Dysplasia of the atrioventricular valves
Dysplasia of the atrioventricular valves is caused by an abnormal development of the atrioventricular cushions that lead to the formation of valve cusps that are too short to fully close the atrioventricular orifice. The condition has been reported in all domestic species. A wide spectrum of lesions has been identified in dogs and cats with atrioventricular valve malformations, including anomalies in valve leaflets, chordae tendineae and/or papillary muscles. Dysplasia of the atrioventricular valves may appear as an isolated lesion or in association with others defects such as subvalvular aortic stenosis (SAS), ventricular septal defects, or atrial septal defects. Cats, and the following dog breeds show a predisposition to mitral dysplasia: Great Danes, German Shepherds, Bull Terriers, Golden Retrievers, Newfoundlands, Dalmatians, and Mastiffs. Tricuspid dysplasia has also been reported in cats. Regurgitation of blood occurs upon systole. This condition is often called left atrioventricular valve insufficiency and usually leads to left heart failure.
Transposition of great vessels
Reversal of systemic and pulmonary outflows is a rare event. On rare occasions the conu-truncal cushions fail to spiral as they divide the outflow tract into two channels. This causes the fourth aortic arches to connect with the right ventricles and the sixth aortic arches with the left ventricle. This results in two totally independent circulatory arcs, with the right ventricle emptying into the aorta and the left ventricles into the pulmonary artery. In such a condition the ventricles are of the same size and generate comparable pressure. Since the systemic and the pulmonary circulations are separate and closed, the presence of left-right shunts in the heart is necessary for postnatal survival. This anomaly is compatible with life only if an atrial and ventricular septal defect and an associated patent ductus arteriosus accompany it, but even with these anatomical compensations, the quality of the blood reaching the body is poor.
Congenital malformations of the vascular system
Persistent right aortic arch
Persistent right aortic arch results from a failure of the right dorsal aorta to degenerate between the seventh dorsal intersegmental artery and the point of fusion of the paired aortae. Persistence of the right aortic arch has been found in cattle, pigs, horses, and cats, and it is fairly common in dogs. It is responsible for 95% of the vascular ring defects in dogs and is most frequently seen in larger breeds (e.g. German Shepherds, Weimeraners, and Irish Setters). This malformation can appear under different phenotypes: in one form, the right arch connection persists and the left one disappears, resulting in the arch of the aorta being on the right instead on the left side; in a second form, both connections persist, but the right one is only a fibrous remnant without a vascular lumen; in a third form, both connections possess the characteristics of a vessel, which results in a double aortic arch.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree