CHAPTER 12 Development of the blood cells, heart and vascular system
During the early phases of development, the embryo is nourished through diffusion from the fluid secreted by the uterine glands into the uterine cavity. However, as the size and complexity of the embryo increases, it soon needs a circulatory system to distribute nutrients and oxygen and to remove carbon dioxide and metabolites. The circulatory system, including the heart, arteries, veins and blood, begins to develop as early as the third week of gestation to meet this need; it is the first functional organ system.
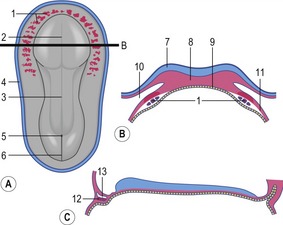
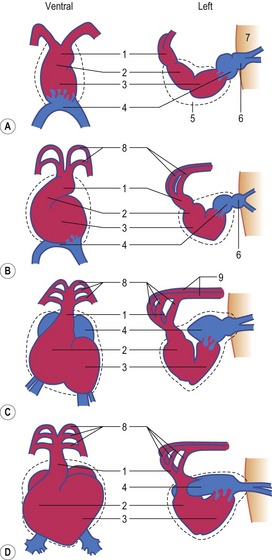
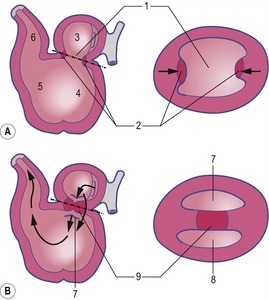
The formation of blood and blood-vessels is initiated from haemangioblasts in the visceral mesoderm of the yolk-sac wall. An area of blood-forming cavities is established in the visceral mesoderm in the anterior part of the embryo where it forms a horseshoe-shaped structure around the anterior and lateral portions of the neural plate (Fig. 12-1). This structure is referred to as the cardiogenic field, and the intra-embryonic coelom overlying it develops into the pericardial cavity. Gradually, the blood-forming cavities coalesce to form a horseshoe-shaped tube, the endocardial tube, lined by endothelial cells. This tube becomes surrounded by myoblasts (which develop from the mesenchyme) to form the myocardium. On the surface of the myocardium the visceral mesoderm of the pericardial cavity forms the epicardium, thereby completing the formation of the cardiac tube.
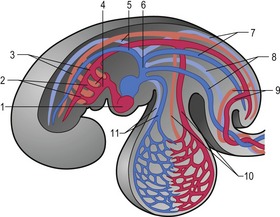
Outside the cardiogenic field, clusters of angioblasts also assemble on each side of the midline of the embryo. These bilateral assemblies develop into tubes lined by endothelial cells – the two dorsal aortae. Antero-posterior folding of the embryo by about 180 degrees at the front end of the embryonic disc translocates the horseshoe-shaped cardiac tube, enclosed in its pericardial cavity, from an anterior to a ventral position (Fig. 12-2). In the process, the posterior extensions of the horseshoe-shaped cardiac tube are transformed into anterior extensions that develop into the two ventral aortae defining the future outlet of the heart (Fig. 12-3). Another result of the antero-posterior folding is that the cardiac tube, with the two ventral aortae extending in an anterior direction, becomes located ventral to the anterior portions of the dorsal aortae. This spatial relationship allows for the dorsal and ventral aortae on each side to become connected by aortic arches that correspond to the pharyngeal or branchial arches (see ‘The aortic arches’, this chapter). The dorsal and ventral aortae, together with the aortic arches, form the backbone of the arterial system whereas the initially curved portion of the cardiogenic field develops into the heart (Fig. 12-4). Along with these processes, bilateral collecting systems, returning blood from the arterial system to the heart, form the basis of the venous system which become connected to the posterior crescent of the horseshoe-shaped cardiac tube defining the future inlet to the heart (Fig. 12-3). In the following, the development of the heart, the arterial and the venous systems will be described in more detail. The lymphatic system is described in Chapter 13.
FORMATION OF BLOOD CELLS
The formation of blood cells, haematopoiesis, occurs in three overlapping periods. The first, or mesoblastic period of blood-formation, occurs in the yolk sac. During the second, or hepato-lienal period, the liver and the spleen become the major blood-forming organs. This period is, in turn, overtaken by a third, or medullary period, in which the bone marrow takes over as the major blood-forming organ.
The mesoblastic period
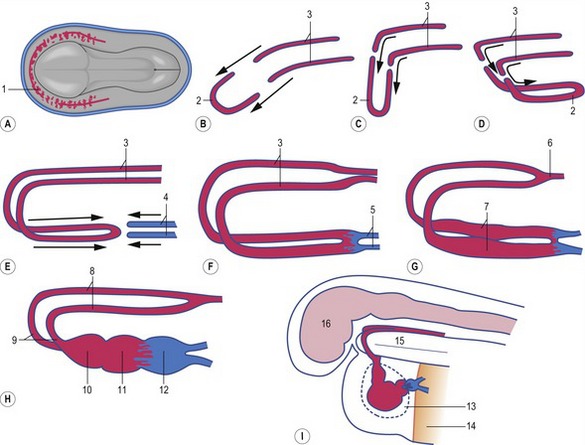
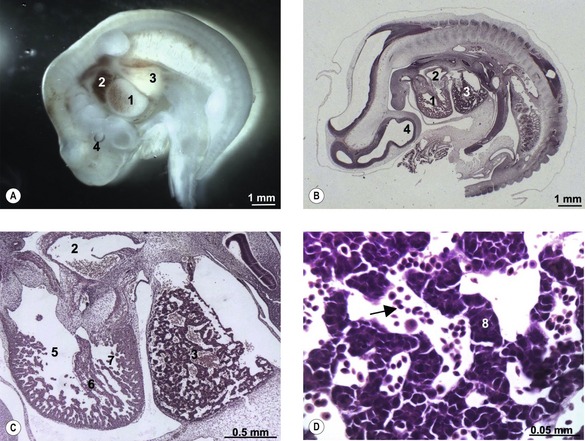
The first blood cells appear in the visceral mesoderm of the yolk-sac wall very early in development (Fig. 12-5); in cattle, they can be seen in embryos at a crown-rump length of 4 mm. At first, blood islands are formed where larger spaces in the mesoderm become occupied with clusters of haemangioblasts, in which the outer cells differentiate into angioblasts, forming endothelial cells, and the inner cells into primitive blood cells. These blood islands coalesce into larger units and the endothelial cells form tubes establishing the first vessels. This process of spontaneous blood-vessel formation is referred to as vasculogenesis. Subsequently, the first generation of vessels forms new vessels by sprouting – a process referred to as angiogenesis. The first blood cells to be formed are primitive nucleated erythrocytes. This primitive erythropoiesis depends on the formation of erythropoietic stem cells that need to be in contact with the hypoblast covering the yolk sac in order to maintain stem cell function. Primitive erythropoiesis evolves within a few days into mature erythropoiesis resulting in erythrocytes without nuclei. In cattle, primitive nucleated erythrocytes constitute 90 to 100% of the erythrocyte population at a crown-rump length of 10 to 16 mm; however, at a length of 23 to 30 mm, their contribution has decreased to 25 to 50%. More recent studies, however, have shown the yolk sac to have only limited haematopoietic potential compared with that appearing shortly after in the intra-embryonic aorta-gonad-mesonephros (AGM) region. It is apparently stem cells from this region that contribute to haematopoiesis in the following two periods.
THE HEART
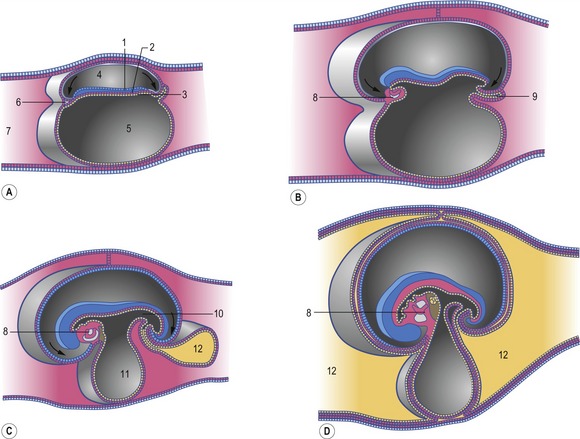
The heart develops from the horseshoe-shaped cardiac tube after embryonic folding has repositioned it within the pericardial cavity ventral to the embryonic disc (Fig. 12-3). The anterior extensions of the horseshoe develop into the two ventral aortae whereas the posterior crescent makes contact with the developing venous system. In parallel with the antero-posterior folding, a lateral folding moves the lateral aspects of the embryonic disc ventrally and towards each other in the midline of the embryo. This folding brings the posterior portions of the two ventral aortae gradually closer to each other ventral to the foregut; eventually portions of the two aortae fuse form to a single tube which extends the cardiac tube anteriorly (Fig. 12-3). At its anterior end, the cardiac tube will be continuous with the two ventral aortae, defining the outlet of the heart; at its posterior end, the tube will be joined by the venous system, defining the inlet of the heart. The tube expands in diameter and begins to pump blood out into the ventral aortae, the aortic arch system and thence the dorsal aortae. In return it receives the venous drainage at its posterior pole. Coordinated embryonic heart beats begin at around Day 22 of pregnancy in the pig, Day 23 in the dog and cattle, and Day 24 in the horse.
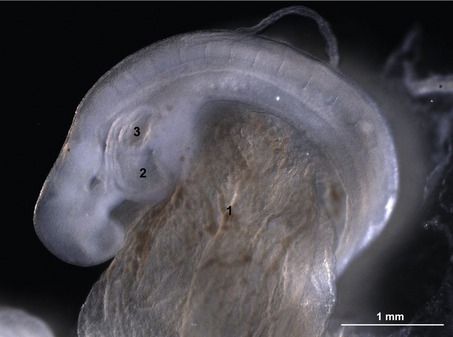
Segmentation of the cardiac tube and loop formation
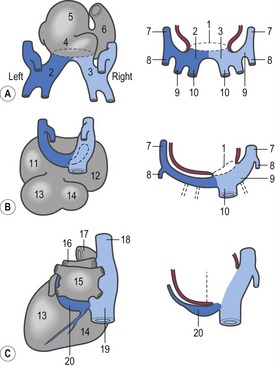
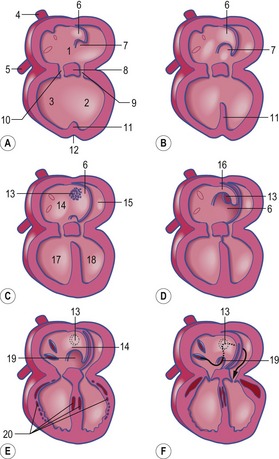
In the pericardial cavity the cardiac tube is suspended in a dorsal mesocardium and anchored to a ventral mesocardium (the latter deteriorates after only a short time). Some portions of the cardiac tube expand more quickly than others, resulting in a segmented tube with dilatations separated by indentations (Fig. 12-3). In posterior-anterior order, the expanded portions of the cardiac tube are the sinus venosus, where the veins open into the cardiac tube, the atrium, the ventricle, the bulbus cordis and the truncus arteriosus, where the outlet into the ventral aortae is found (Figs 12-6, 12-7). The truncus arteriosus is formed from cells of neural crest origin. The expanded portions of the cardiac tube are connected by narrower channels. Because the cardiac tube outgrows the pericardial cavity, and because the tube is fixed in the pericardium at both ends, the tube becomes U-shaped with the loop of the U (the junction between the ventricle and the bulbus cordis) pointing ventrally. The loop is prominent and forms the heart bulge that is clearly seen on the outside of the embryo and is characteristic of this stage of development. At least in cattle, the loop formation occurs around the 10–12 somite stage, around Day 22 of gestation. Throughout this process, the developing heart is beating at a rhythm set by pacemakers in the sinus venosus. At first, the sinus venosus and the atrium are not enclosed within the pericardial cavity, but they gradually become enveloped by the pericardium. During this enclosure, the atrium becomes positioned dorsal to the ventricle, and the loop takes on the shape of an S instead of a U. Again in cattle, this process occurs around the 20 somite stage, i.e. around Day 23 of gestation. The tube is smooth-walled as it begins its loop formation, but then regions on either side of the primary interventricular foramen (between the ventricle and the bulbus cordis) develop trabeculae. The trabeculated portion of the ventricle develops into the future left ventricle whereas the trabeculated portion of the bulbus cordis develops into the future right ventricle.
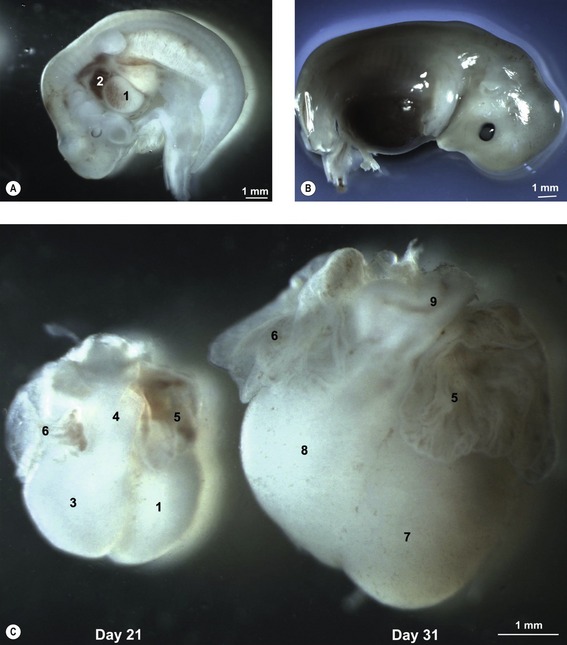
Fig. 12-7: Heart development in pig embryos at Day 21 (A) and 31 (B) of development. A: 1: Ventricle; 2: Atrium. C: Left aspect of hearts from Day 21 and 31 pig embryos. 1: Ventricle; 3: Bulbus cordis; 4: Truncus arteriosus; 5: Left auricle; 6: Right auricle; 7: Left ventricle; 8: Right ventricle; 9: Conus arteriosus.
Formation of the four heart chambers
Incorporation of the sinus venosus into the atrium
The first veins to open into the sinus venosus are the ompholomesenteric or vitelline veins (see ‘Development of the venous system’, later in this chapter), followed shortly by the umbilical and the cardinal veins. The three pairs of veins are connected with the sinus venosus in such a manner that the sinus forms right and left sinus horns (Fig. 12-8). Gradually, the right side of the venous inlet is favoured and the opening from the sinus venosus into the atrium shifts to the right and becomes narrower. During this process, a portion of the right part of the sinus venosus (the right sinus horn) merges into the atrium (Fig. 12-9). The lateral crest-shaped borderline between the incorporated sinus and the atrium develops into the crista terminalis whereas the medial one develops into the septum secundum (see ‘Division of the atrium’, later in this chapter). The anterior portion of the right sinus horn develops into the part of the cranial vena cava that opens into the right atrium whereas the posterior portion develops into the corresponding portion of the caudal vena cava. The left sinus horn eventually develops into the coronary sinus. The original right atrium remains as the right auricle whereas the smooth-walled portion of the atrium develops from the incorporated sinus venosus.
While the sinus venosus is being incorporated into the right side of the atrium, the pulmonary veins start to open into the left side of the atrium. At first, they do so through a single opening from the common cavity into which four pulmonary veins drain. Later, however, the common cavity is incorporated into the atrium resulting in four individual openings for the pulmonary veins (Fig. 12-9). The original left atrium remains as the left auricle whereas the smooth-walled portions of the atrium are formed by the incorporated parts of the pulmonary veins.
Division of the atrioventricular channel
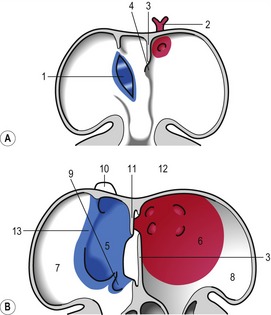
At the level of the atrioventricular channel, the inner cardiac wall develops anterior and posterior thickenings, the endocardial cushions, which grow until they meet and fuse in the midline of the canal to form the septum intermedium (Fig. 12-10). This divides the channel into right and left atrioventricular channels.
Division of the atrium
To separate the blood circulations to the body and lungs, the cardiac tube derivatives need to be divided into right and left compartments. As the septum intermedium develops from the endocardial cushions, a crescent-shaped fold, the septum primum, develops from the dorsal aspect of the atrium and separates it into right and left components connected only through a minor opening, the ostium primum (Fig. 12-11). Around this ostium, the septum primum extends all the way to the endocardial cushions, thus contributing to the development of the septum intermedium. As the endocardial cushions grow towards the midline, the septum primum also grows, and when the cushions fuse, the septum also fuses ventrally, thus gradually closing the ostium primum. Before this closure, however, programmed cell death in the dorsal region of septum primum results in formation of the ostium secundum which allows continued blood flow from the developing right atrium to the left (Fig. 12-11). In the horse, this event occurs at a crown-rump length of 11.5–12 mm, i.e. around Day 30-32 of gestation. Shortly thereafter, a second crescent-shaped fold, the septum secundum, develops to the left of the septum primum. The septum secundum grows until it has completely covered the ostium secundum, but retains an oval opening, the foramen ovale. The dorsal portion of the septum secundum fuses with the septum primum whereas the ventral portion establishes a valve regulating the blood flow from the primitive right atrium to the left atrium.
Division of the ventricle and the bulbus cordis
When the U-shaped cardiac tube is formed, the junction between the ventricle and the bulbus cordis points ventrally. The bulbus cordis consists of a dilated portion adjacent to the ventricle and a narrower portion, the conus cordis, joining the truncus arteriosus. The transition between the ventricle and the bulbus cordis is marked externally by a groove and internally by a muscular fold that develops into the muscular part of the interventricular septum, which grows dorsally towards the septum intermedium (Figs 12-11, 12-12). An interventricular foramen persists for some time, but this opening is gradually closed by the membranous part of the interventricular septum which develops from the posterior endocardial cushion. In the horse at least, the closure of the interventricular septum occurs at a crown-rump length of 14 to 16 mm, i.e. around Day 35–36 of gestation. It is important to understand that the membranous part of the interventricular septum develops in such a way that both the ventricle (the future left ventricle) and the dilated portion of the bulbus cordis (the future right ventricle) maintain openings into the conus cordis. In parallel with the septum formation, the walls of the ventricle and the bulbus cordis thicken and develop a trabeculated pattern on the inside. Trabeculation results to some degree from the formation of cavitations in the wall which later open into the lumen. The expansion of the ventricle and the bulbus cordis brings their walls into apposition ventrally. There, the walls gradually fuse and add to the interventricular septum (Fig. 12-6).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree