(1)
Arezzo, Italy
2.1 Introduction
There are many skin lesions and they differ from one another morphologically. Papules, pustules, epidermal collarettes, scales, erosions, ulcers, plaques, nodules and swellings are clinically easy to recognise. Some of these lesions may be present simultaneously in the same patient, representing different stages of the same disease or clinical signs of different coexisting diseases.
Cytology is one of the fastest, most inexpensive and easy to perform of the diagnostic techniques available. As with all diagnostic tests, the quality of the specimens is basic for correct interpretation; even if more than one sampling technique can be performed from the same lesion, there is always one that is most suitable, offering more guarantees of obtaining a representative sample. This chapter discusses the sampling methods in canine and feline skin cytology. Starting from clinical lesions, the reader is provided with fundamental information for a correct preparation and staining of slides.
Based on the above, the execution of a correct cytological sampling cannot disregard three basic steps:
- (a)
The choice of the lesion to be sampled
- (b)
The choice of the sampling method most suitable for that particular lesion
- (c)
Proper preparation of the slide
2.2 Sampling Techniques
In practice, the most commonly used sampling techniques include the impression smear, the fine needle biopsy, with or without aspiration, and the scraping (Raskin and Meyer 2015; Valenciano and Cowell 2014).
2.2.1 Impression Smears
The impression smear is the only executable method we can perform when cells must be sampled from non-raised, superficial “wet” (exudative) lesions. This technique is frequently used because with this method, it is possible to collect cells simply by placing a slide on the lesion. As the cells easily adhere to the slide, it is sufficient to gently place it on the lesion without exerting excessive pressure, which could alter the cell morphology. Nevertheless, there are some basic precautions that differ according to the lesion that must be investigated. The skin lesions from which cells can be obtained using the impression smears technique are numerous and are covered below.
2.2.1.1 Cytological Sampling from Papules
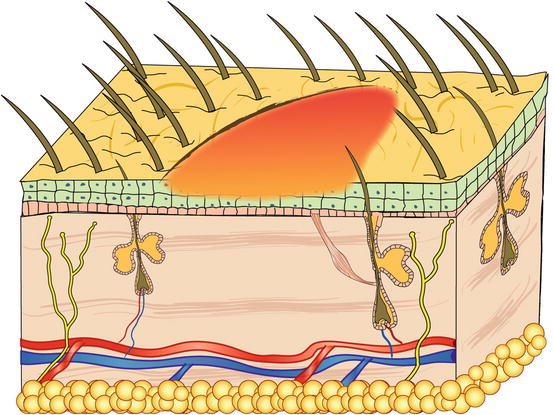
Papules are small, solid, raised and palpable primary lesions, usually erythematous, smaller than 1 cm (Figs. 2.1 and 2.2). Histopathologically, papules are composed of inflammatory or, exceptionally, neoplastic cells, located in the superficial dermis and usually cause a slight hyperplasia of the overlying epidermis. Intra-epidermal exocytosis of inflammatory cells or small ulceration of the epidermis, the latter often located on the top of the papule, cause discharge of a small amount of exudate that, once dehydrated, gives rise to a small crust (Fig. 2.3). These types of lesions are called crusted papules and are frequently observed in cats with miliary dermatitis and in dogs with scabies.
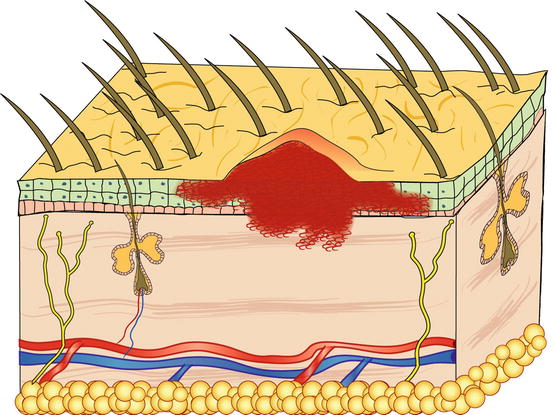
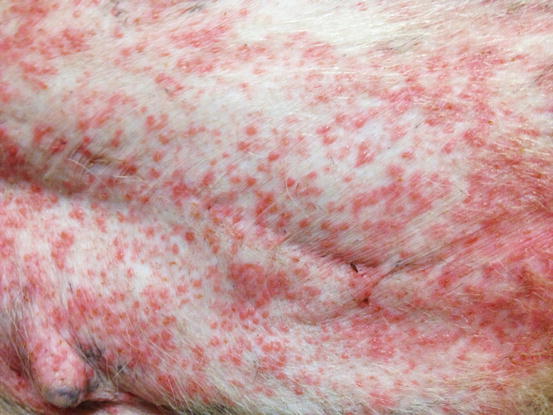
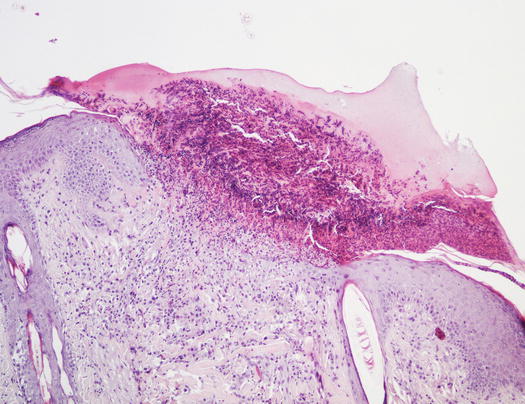

Fig. 2.1
Schematic representation of a papule

Fig. 2.2
Multiple erythematous papules on the abdomen of a dog with superficial pyoderma

Fig. 2.3
Histology of a crusted papule: note the crust covering the ulcer
Papules with an intact surface are too small for FNB and cells cannot be collected using the imprint technique; therefore, only the papular crusted lesions permit the collection of a few cells using this method.
Some dermatologists use the term nodular papule to describe a lesion that is raised and round in shape, which is of intermediate size between a papule and a nodule. Nodular papules are less than 1 cm and even if they can sometimes slightly exceed this size, they do not appear large enough to justify the definition of nodules (Fig. 2.4). These lesions, compared with papules, provide more diagnostic specimens, because through FNB they can supply sufficient cells.
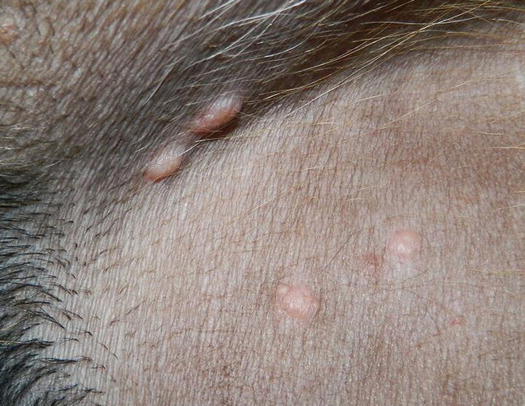

Fig. 2.4
Papular nodules in a dog with the papular form of leishmaniasis
The author prefers to treat the cytological findings of papular nodular lesions separately from those of papules, as he believes that the former are characteristic of some skin diseases and for this reason, deserve a more detailed cytological description.
Sampling Technique
In general, papules do not yield many cells, but, in some cases, even the few cells collected may be useful in the interpretation of the lesions.
In the case of papules with an intact epidermis, the collection of cells is not possible using an impression smear. When the papules ulcerate or when spongiosis promotes the leukocytic exocytosis through the epidermis, it is possible to collect some cells by gently placing a slide on top. To collect cells from crusted papules, the superficial crust must be removed before placing the slide on the exposed exudate (Fig. 2.5). In the case of nodular papules, it is possible try collecting cells via FNB using a small needle (23 G), a technique that will be discussed later.
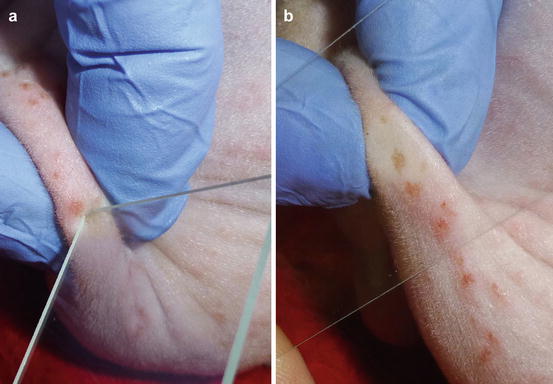

Fig. 2.5
(a) Removal of a crust with a slide, (b) placing the slide onto the exposed exudate
2.2.1.2 Cytological Sampling from Pustules
Pustules are raised, soft, yellowish, intra-epidermal or intra-follicular skin lesions. The yellowish colour of the pustules is closely related to their content of granulocytes, both neutrophils and eosinophils, which are the main cells that comprise the purulent exudate (Figs. 2.6 and 2.7).
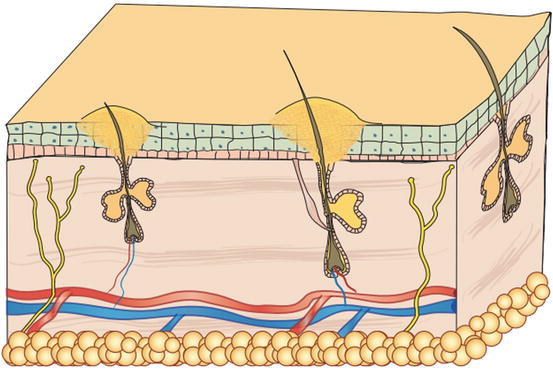
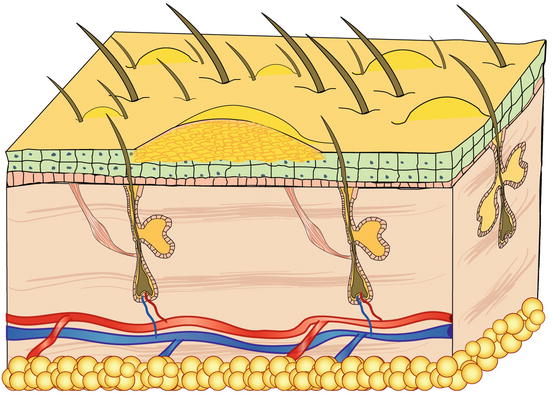

Fig. 2.6
Schematic representation of a follicular pustule

Fig. 2.7
Schematic representation of a non-follicular pustule
By definition, pus is an inflammatory exudate composed of karyolytic neutrophils, necrotic debris and bacteria; according to this definition, the term pus should only be used to describe the content of pustules from pyoderma. As there are many diseases that produce a sterile purulent exudate, the term pus should refer only to its granulocytic composition, and should not imply a bacterial infection.
In addition to granulocytes, pustules may contain other cell types (keratinocytes), microorganisms (bacteria), parasites (Demodex spp.) etc.. The detection of these components in cytological samples provides important diagnostic clues.
Clinically, pustules can be divided into follicular and non-follicular. Follicular pustules are characterised by pus located in the follicular lumen and are clinically recognisable by their small size and by a hair shaft protruding from its centre. As follicular pustules are very small, they are always round and less than 1 cm in size (Fig. 2.8). The term follicular pustule is also used in histopathology to describe an intramural follicular sterile pustule, sometimes observed in pemphigus foliaceus. In these cases, the pustules are so small that they are not clinically detectable; thus, when a hair emerges from a pustule, infectious folliculitis should be suspected (Fig. 2.9).
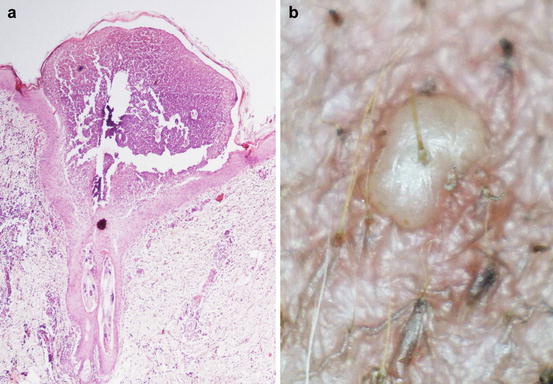
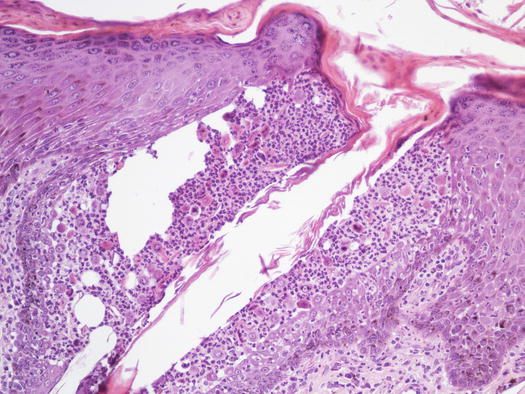

Fig. 2.8
(a) Histology of a follicular pustule: note how the pustule is located on the centre of the follicle; (b) clinical aspect of a follicular pustule: a hair shaft emerges from the centre of the pustule

Fig. 2.9
Histology of two intramural acantholytic follicular pustules in course of pemphigus foliaceus. Note how the pustules are confined in the follicular wall and, as they do not emerge from the center of the follicle, they are not macroscopically evident
In non-follicular pustules, pus is not present in the follicular lumen, but it is intra-epidermal and more frequently intra-corneal (Fig. 2.10). Depending on their extent, non-follicular pustules can be of variable size, round or irregular in shape. They may be small and localised between follicles or so large that they inevitably encompass many hairs, ranging from a few millimetres to over 2 cm (Fig. 2.11). The clinical aspect and localisation of pustules not has only a descriptive meaning, it may also help the clinician to better interpret the cytological results.
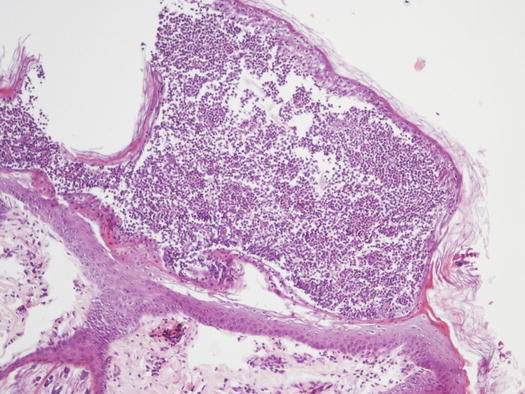
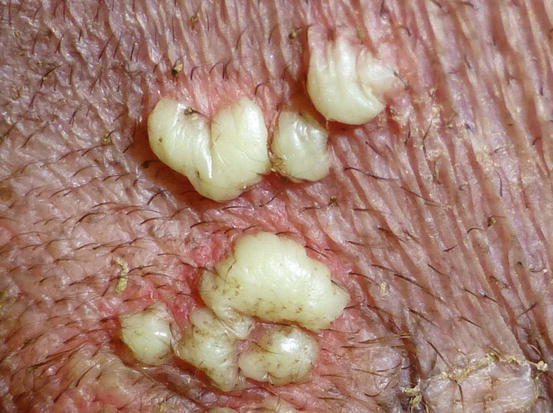

Fig. 2.10
Histology of a non-follicular pustule: note how the pustule does not involve the follicle

Fig. 2.11
Large and non-follicular pustules of irregular shapes in a dog with juvenile impetigo
Sampling Technique
Pustules may be the most important lesions in canine skin cytology, as they usually represent the direct action of a disease or a microorganism. In the case of large pustules, cells can be collected using the impression smear technique: breaking the base of the pustule with a fine needle and lifting the keratinocytes that form its “roof”. The pus is exposed and a sample can be taken by gently placing a slide. This method is a variant of the technique conceived by Dr. Arnault Tzanck, a French dermatologist who was a pioneer of diagnostic cytology in the early 20th century and for this reason, the technique is known as the Tzanck test (Fig. 2.12).
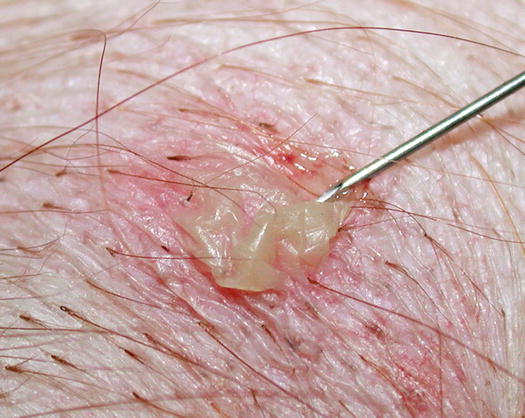

Fig. 2.12
The Tzanck test: rupture of a large pustule with a needle
When pustules are very small, like follicular pustules, this method is very difficult to perform and the risk of penetrating the dermis with the needle and causing bleeding is very high. In these cases, the shorter part of the slide must be slightly and laterally pushed against the base of the pustule, allowing the pus to be transferred onto it (Fig. 2.13).
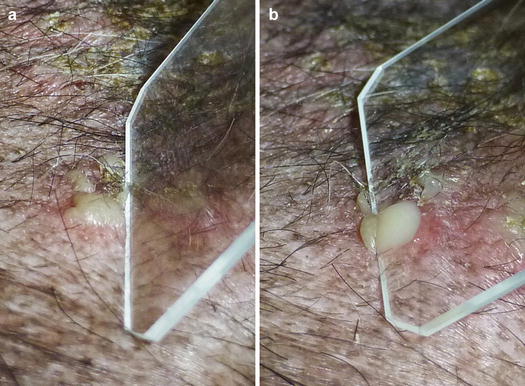

Fig. 2.13
(a) Lateral pressure placed on a small pustule with a slide; (b) the pus is transferred onto the slide
Once collected, the material must be gently smeared onto another slide and left to dry. Pustules tend to rapidly dehydrate and give rise to yellowish crusts or epidermal collarettes. In these cases, as described below, it is possible to obtain cytological specimens by collecting the exudate present below the crust at the edges of the collarettes.
2.2.1.3 Cytological Sampling from Intact Skin Covered with “Dry” or “Oily/Waxy” Scales
Scales are aggregates of corneocytes that represent the final product of the physiological keratinisation process of the epidermis and are continuously released into the environment, both singly and in small clusters (Fig. 2.14). When scales are visible to the naked eye, the clinical finding is described as exfoliative or desquamative dermatitis, which is observed in many canine and feline skin diseases. Scaling diseases recognise both primary and secondary causes; primary causes are usually linked to genetic or idiopathic disorders and in these cases, cytology has no diagnostic interest. Scales are also very common in secondary keratinisation defects, usually caused by infectious (leishmaniasis, dermatophytosis, pyoderma, Malassezia spp.), parasitic (demodicosis), and autoimmune diseases (pemphigus foliaceus), in addition to neoplasia (epitheliotropic lymphoma), whose cytology provides diagnostic information or can direct clinicians in selecting additional diagnostic tests.
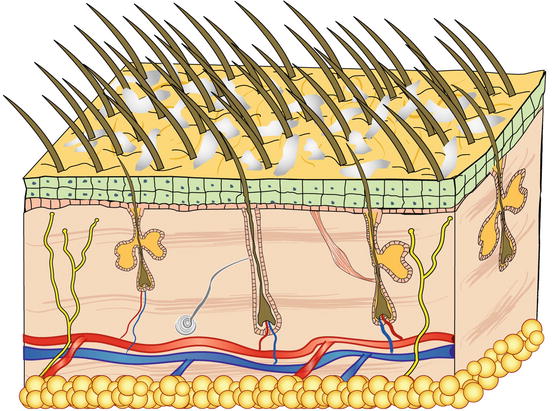

Fig. 2.14
Schematic representation of scales
Sampling Technique
Placing a slide onto an oily/waxy skin allows keratinocytes and different microorganisms present on their surface to be collected. In patients with dry scaly dermatitis, the impression smear technique does not permit the cells to adhere to the slide; in these cases, sampling requires the use of a piece of transparent acetate tape (Fig. 2.15). This method is very useful when searching for yeasts and dermatophytes, but it can be used to collect any type of cell or parasite present on the skin surface, as will be discussed in the Chap. 3.
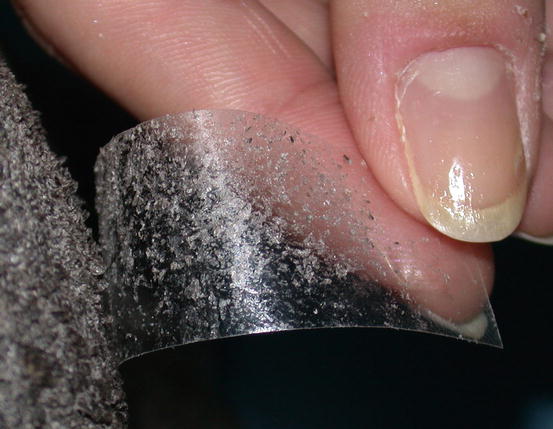

Fig. 2.15
Many scales remain stuck to the adhesive acetate tape
2.2.1.4 Cytological Sampling from Erosions
Erosions are superficial lesions characterised by the loss of the outer layers of the epidermis and do not involve the dermis (Fig. 2.16). Determining the integrity of the basement membrane is not always clinically possible, particularly when the erosion is deep and its base is composed only of the basal layer of the epidermis. Cytological examination confirms or rule outs an ulcer, based on the presence or absence of red blood cells respectively.